Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Antiprotozoal (malaria).
Preparation
Quinine Dihydrochloride Infusion
DEFINITION
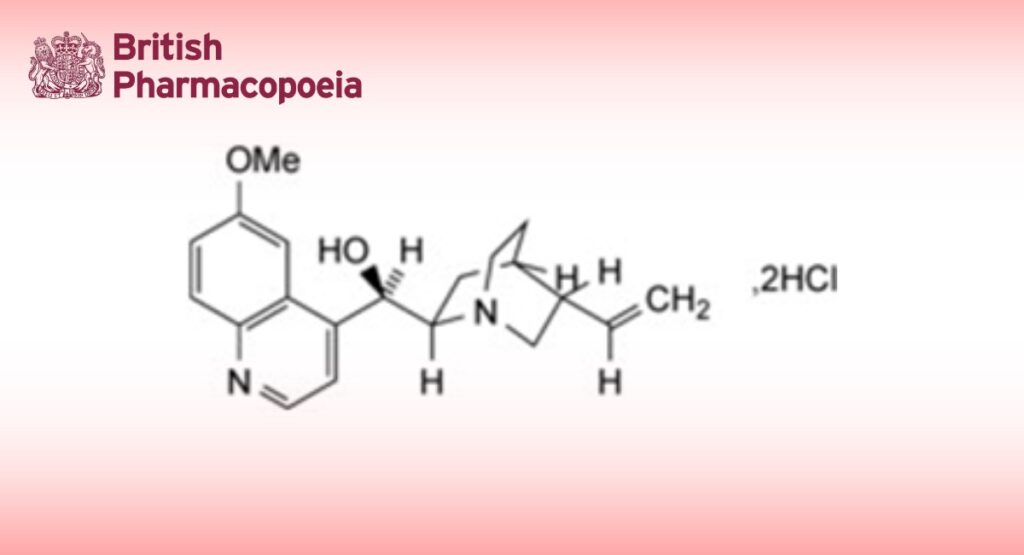
Quinine Dihydrochloride is (8S,9R)-6′-methoxycinchonan-9-ol dihydrochloride. It contains not less than 99.0% and not more than 101.0% of alkaloid dihydrochlorides, calculated as C20H24N2O2,2HCl, with reference to the dried substance.
CHARACTERISTICS
A white or almost white powder.
Very soluble in water; soluble in ethanol (96%).
IDENTIFICATION
A. Carry out the method for thin-layer chromatography (2.2.27), Appendix III A, using the following solutions in methanol.
(1) 1.0% w/v of the substance being examined.
(2) 1.0% w/v of quinine sulfate BPCRS.
(3) 1.0% w/v each of quinidine sulfate BPCRS and quinine sulfate BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel G.
(b) Use the mobile phase as described below.
(c) Apply 4 µL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry it in a current of air for 15 minutes and repeat the development. Dry the plate at 105° for 30 minutes, allow to cool and spray with iodoplatinate reagent.
MOBILE PHASE
15 volumes of diethylamine, 36 volumes of ether and 60 volumes of toluene.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (3) shows two clearly separated spots.
CONFIRMATION
The principal spot in the chromatogram obtained with solution (1) corresponds in position, colour and size to that in the chromatogram obtained with solution (2).
B. Complies with the test for Acidity.
C. Yields reaction A characteristic of chlorides, (2.3.1) Appendix VI.
TESTS
Acidity
pH of a 3% w/v solution, 2.0 to 3.0, Appendix V L.
Specific optical rotation (2.2.7)
In a 3% w/v solution in 0.1M hydrochloric acid, -223 to -229 calculated with reference to the dried substance, Appendix V F.
Barium
To 15 mL of a 2.0% w/v solution add 1 mL of 1M sulfuric acid. The solution remains clear for at least 15 minutes.
Sulfate (2.4.13)
0.125 g complies with the limit test for sulfates, Appendix VII (0.12%).
Other cinchona alkaloids
Carry out the method for liquid chromatography (2.2.29) Appendix III D, using the normalisation procedure and the following solutions in the mobile phase.
(1) Dissolve 20 mg of the substance being examined, with gentle heating if necessary, in 5 mL and dilute to 10 mL.
(2) Prepare as for solution (1) but using quinine sulfate BPCRS in place of the substance being examined.
(3) Prepare as for solution (1) but using quinidine sulfate BPCRS in place of the substance being examined.
(4) Mix equal volumes of solutions (2) and (3).
(5) Dilute 1 volume of solution (2) to 10 volumes and dilute 1 volume of the resulting solution to 50 volumes.
(6) 0.10% w/v of thiourea.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Hypersil ODS 5 µm is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 250 nm for recording the chromatogram obtained with solution (6) and a detection wavelength of 316 nm for the other solutions.
(f) Inject 10 µL of each solution. Inject separately 10 µL of each of solutions (3) and (6).
(g) For solution (1) allow the chromatography to proceed for 2.5 times the retention time of the principal peak.
MOBILE PHASE
Dissolve 6.8 g of potassium dihydrogen orthophosphate and 3.0 g of hexylamine in 700 mL of water, adjust to pH 2.8 with 1M orthophosphoric acid, add 60 mL of acetonitrile and dilute to 1000 mL with water. If necessary adjust the concentration of acetonitrile in the mobile phase so that in the chromatogram obtained with solution (3) the capacity factor of the peak due to quinidine is 3.5 to 4.5, VO being calculated from the peak due to thiourea in the chromatogram obtained with
solution (6).
SYSTEM SUITABILITY
The test is not valid unless (a) in the chromatogram obtained with solution (4) the resolution between the peaks due to quinine and quinidine is at least 1.5 and the resolution between the peaks due to dihydroquinidine and quinine is at least 1.0 and (b) the signal-to-noise ratio of the principal peak in the chromatogram obtained with solution (5) is at least 5.
The chromatogram obtained with solution (2) shows a principal peak due to quinidine and a peak due to dihydroquinine with a retention time relative to quinine of about 1.4. The chromatogram obtained with solution (3) shows a principal peak due to quinidine and a peak due to dihydroquinidine, with a retention time relative to quinidine of about 1.2. The chromatogram obtained with solution (4) shows four peaks due to quinine, dihydroquinine, quinidine and dihydroquinidine which are identified by comparison of their retention times with those of the corresponding peaks in the chromatograms obtained with solutions (2) and (3).
LIMITS
In the chromatogram obtained with solution (1):
the content of dihydroquinine is not greater than 10%;
the content of any related substance eluting before quinine is not more than 5%; the content of any other related substance is not more than 2.5%.
Disregard any peak with an area less than that of the peak in the chromatogram obtained with solution (5).
Loss on drying (2.2.32)
When dried to constant weight at 105°, loses not more than 3.0% of its weight. Use 1 g.
Sulfated ash (2.4.14)
Not more than 0.1%, Appendix IX A.
ASSAY
Dissolve 0.150 g in a mixture of 10 mL of water and 50 mL of ethanol. Titrate with 0.1M sodium hydroxide, determining the end-point potentiometrically, Appendix VIII B.
Each mL of 0.1M sodium hydroxide is equivalent to 39.73 mg of C20H24N2O2,2HCl.
STORAGE
Quinine Dihydrochloride should be protected from light.