Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Class I antiarrhythmic.
Preparation
Quinidine Sulfate Tablets
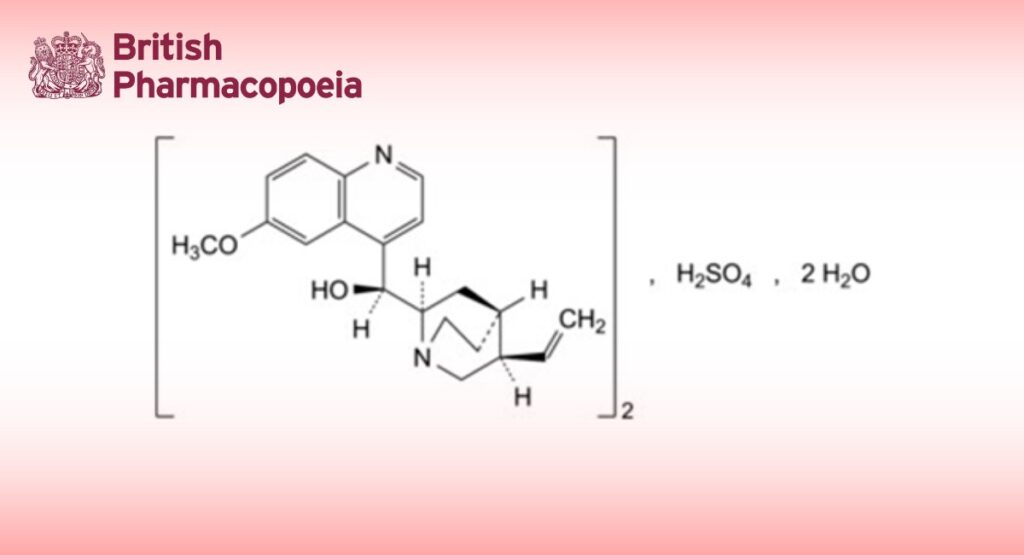
DEFINITION
Alkaloid monosulfates, expressed as bis[(S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]oct-2-yl](6-methoxyquinolin-4- yl)methanol] sulfate dihydrate.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or silky, colourless needles.
Solubility
Slightly soluble in water, soluble in boiling water and in ethanol (96 per cent), practically insoluble in acetone.
IDENTIFICATION
A. Thin-layer chromatography (2.2.27).
Test solution Dissolve 0.10 g of the substance to be examined in methanol R and dilute to 10 mL with the same solvent. Reference solution Dissolve 0.10 g of quinidine sulfate CRS in methanol R and dilute to 10 mL with the same solvent. Plate TLC silica gel G plate R.
Mobile phase diethylamine R, ether R, toluene R (10:24:40 V/V/V). Application 5 µL.
Development Twice over a path of 15 cm; dry in a current of air for 15 min between the 2 developments.
Drying At 105 °C for 30 min and allow to cool.
Detection Spray with iodoplatinate reagent R.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
B. Dissolve about 5 mg in 5 mL of water R. Add 0.2 mL of bromine water R and 1 mL of dilute ammonia R2. A green colour develops.
C. Dissolve 0.1 g in 3 mL of dilute sulfuric acid R and dilute to 100 mL with water R. When examined in ultraviolet light at 366 nm, an intense blue fluorescence appears which disappears almost completely on addition of 1 mL of hydrochloric acid R.
D. Dissolve about 50 mg in 5 mL of hot water R, cool, add 1 mL of silver nitrate solution R1 and stir with a glass rod. After a few minutes, a white precipitate is formed that dissolves on the addition of dilute nitric acid R.
E. It gives reaction (a) of sulfates (2.3.1).
F. pH (see Tests).
TESTS
Solution S
Dissolve 0.500 g in 0.1 M hydrochloric acid and dilute to 25.0 mL with the same acid.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution GY6 (2.2.2, Method II).
pH (2.2.3)
6.0 to 6.8.
Dissolve 0.10 g in carbon dioxide-free water R and dilute to 10 mL with the same solvent.
Specific optical rotation (2.2.7)
+ 275 to + 290 (dried substance), determined on solution S.
Other cinchona alkaloids
Liquid chromatography (2.2.29): use the normalisation procedure.
Test solution Dissolve 20 mg of the substance to be examined in 5 mL of the mobile phase, with gentle heating if necessary, and dilute to 10 mL with the mobile phase.
Reference solution (a) Dissolve 20 mg of quinine sulfate CRS (impurity A) in 5 mL of the mobile phase, with gentle heating if necessary, and dilute to 10 mL with the mobile phase.
Reference solution (b) Dissolve 20 mg of quinidine sulfate CRS in 5 mL of the mobile phase, with gentle heating if necessary, and dilute to 10 mL with the mobile phase.
Reference solution (c) To 1 mL of reference solution (a) add 1 mL of reference solution (b).
Reference solution (d) Dilute 1.0 mL of reference solution (a) to 10.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 50.0 mL with the mobile phase.
Reference solution (e) Dissolve 10 mg of thiourea R in the mobile phase and dilute to 10 mL with the mobile phase.
Column:
— size: l = 0.15-0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5-10 µm).
Mobile phase Dissolve 6.8 g of potassium dihydrogen phosphate R and 3.0 g of hexylamine R in 700 mL of water R, adjust to pH 2.8 with dilute phosphoric acid R, add 60 mL of acetonitrile R and dilute to 1000 mL with water R.
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 250 nm for reference solution (e) and at 316 nm for the other solutions.
Injection 10 µL.
Run time 2.5 times the retention time of quinidine.
Identification of peaks Use the chromatogram obtained with reference solution (a) to identify the peaks due to impurity A and dihydroquinine; use the chromatogram obtained with reference solution (b) to identify the peaks due to quinidine and impurity C; the chromatogram obtained with reference solution (c) shows 4 peaks due to quinidine, impurity A, impurity C and dihydroquinine which are identified by comparison of their retention times with those of the corresponding peaks in the chromatograms obtained with reference solutions (a) and (b).
Relative retention With reference to impurity A: dihydroquinine = about 1.4.
Relative retention With reference to quinidine: impurity C = about 1.5.
System suitability:
— resolution: minimum 3.0 between the peaks due to impurity A and quinidine and minimum 2.0 between the peaks due to impurities C and A in the chromatogram obtained with reference solution (c);
— signal-to-noise ratio: minimum 4 for the principal peak in the chromatogram obtained with reference solution (d);
— mass distribution ratio: 3.5 to 4.5 for the peak due to quinidine in the chromatogram obtained with reference solution (b), tR′ being calculated from the peak due to thiourea in the chromatogram obtained with reference solution (e); if necessary, adjust the concentration of acetonitrile in the mobile phase.
Limits:
— impurity C: maximum 15 per cent;
— any impurity eluted before quinidine: for each impurity, maximum 5 per cent;
— any other impurity: for each impurity, maximum 2.5 per cent;
— disregard limit: the area of the principal peak in the chromatogram obtained with reference solution (d) (0.2 per cent).
Boron
Maximum 5 ppm. Avoid where possible the use of glassware.
Test solution Dissolve 1.00 g in a mixture of 0.5 mL of hydrochloric acid R and 4.0 mL of water R.
Reference solution Dissolve 0.572 g of boric acid R in water R and dilute to 1000.0 mL with the same solvent. Dilute 5.0 mL of the solution to 100.0 mL with water R.
To 1.0 mL of this solution add 3.0 mL of water R and 0.5 mL of hydrochloric acid R.
Blank solution Add 0.5 mL of hydrochloric acid R to 4.0 mL of water R.
Add 3.0 mL of a 100 g/L solution of 2-ethylhexane-1,3-diol R in methylene chloride R to the test solution, to the reference solution and to the blank solution, then shake for 1 min. Allow to stand for 6 min. To 1.0 mL of the lower layer, add 2.0 mL of a 3.75 g/L solution of curcumin R in anhydrous acetic acid R and 0.3 mL of sulfuric acid R. Mix and after 20 min add 25.0 mL of ethanol (96 per cent) R. Mix. The blank solution is yellow. Any red colour in the test solution is not more intense than that in the reference solution.
Loss on drying (2.2.32)
3.0 per cent to 5.0 per cent, determined on 1.000 g by drying in an oven at 130 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.200 g in 20 mL of acetic anhydride R. Titrate with 0.1 M perchloric acid, using 0.15 mL of naphtholbenzein solution R as indicator.
1 mL of 0.1 M perchloric acid is equivalent to 24.90 mg of C40H50N4O8S.
STORAGE
Protected from light.
IMPURITIES
A. (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]oct-2-yl](6-methoxyquinolin-4-yl)methanol (quinine),
B. (S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]oct-2-yl](quinolin-4-yl)methanol (cinchonine),
C. (S)-[(2R,4S,5R)-5-ethyl-1-azabicyclo[2.2.2]oct-2-yl](6-methoxyquinolin-4-yl)methanol (dihydroquinidine).