Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Anthelminthic.
DEFINITION
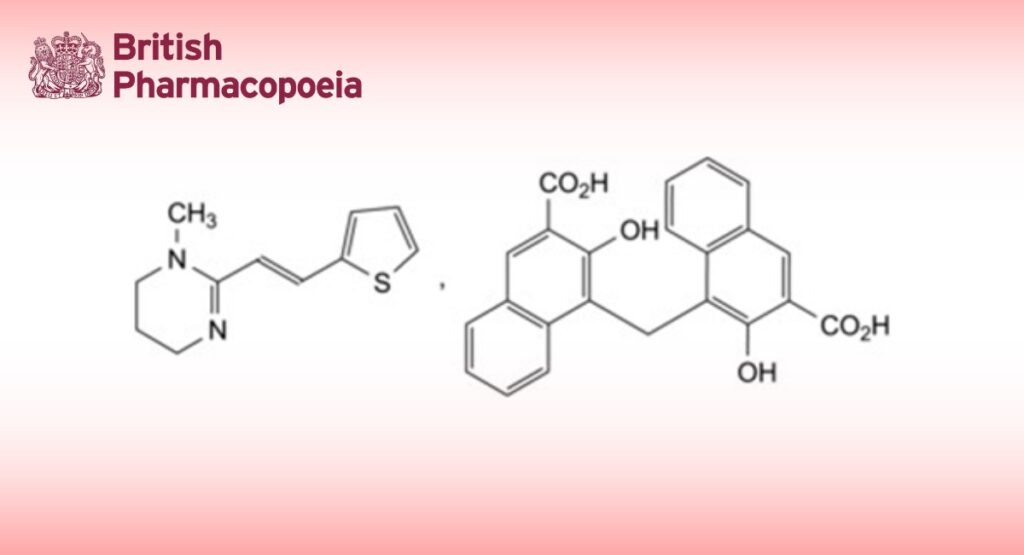
1-Methyl-2-[(E)-2-(thiophen-2-yl)eth-1-en-1-yl]-1,4,5,6-tetrahydropyrimidine hydrogen 4,4′-methylenebis(3- hydroxynaphthalene-2-carboxylate).
Content
98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
Pale yellow or yellow powder.
Solubility
Practically insoluble in water, soluble in dimethyl sulfoxide, practically insoluble in methanol.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison pyrantel embonate CRS.
TESTS
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use and protect from light.
Solvent mixture Mix 5 volumes of glacial acetic acid R and 5 volumes of water for chromatography R, then add 2 volumes of diethylamine R with cooling.
Test solution (a) Dissolve 0.800 g of the substance to be examined in 7 mL of the solvent mixture and dilute to 100.0 mL with acetonitrile R.
Test solution (b) Dilute 1.0 mL of test solution (a) to 10.0 mL with the mobile phase.
Reference solution (a) Dissolve 10 mg of pyrantel impurity A CRS in the solvent mixture, add 2.5 mL of test solution (b) and dilute to 50 mL with the solvent mixture.
Dilute 2 mL of this solution to 100 mL with the solvent mixture.
Reference solution (b) Dilute 1.0 mL of test solution (b) to 200.0 mL with the mobile phase.
Reference solution (c) Dissolve 8.0 mg of pyrantel impurity D CRS in acetonitrile R and dilute to 10.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with acetonitrile R.
Reference solution (d) Dissolve 8.0 mg of pyrantel impurity C CRS in acetonitrile R and dilute to 10.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with acetonitrile R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: silica gel for chromatography R (5 µm);
— temperature: 30 °C.
Mobile phase Solvent mixture, acetonitrile for chromatography R (72:928 V/V). Flow rate 1 mL/min.
Detection Spectrophotometer at 288 nm and, for impurity D, at 238 nm.
Injection 20 µL of test solution (b) and reference solutions (a), (b) and (d); for impurity D, 50 µL of test solution (a) and reference solution (c).
Run time 4 times the retention time of pyrantel.
Identification of impurities Use the chromatogram obtained with reference solution (d) to identify the peak due to impurity C; use the chromatogram obtained with reference solution (c) to identify the peak due to impurity D.
Relative retention With reference to pyrantel (retention time = about 11 min): impurity C = about 0.3; embonic acid = about 0.5; impurity A = about 1.3; impurity D = about 2.2.
System suitability Reference solution (a):
— resolution: minimum 4.0 between the peaks due to pyrantel and impurity A.
Calculation of percentage contents:
— for impurity D, use the concentration of impurity D in reference solution (c);
— for impurity C, use the concentration of impurity C in reference solution (d);
— for impurities other than C and D, use the concentration of pyrantel embonate in reference solution (b).
Limits:
— impurity D: maximum 0.2 per cent;
— impurity C: maximum 0.10 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— sum of impurities other than C and D (excluding embonic acid): maximum 0.3 per cent;
— reporting threshold: 0.05 per cent.
Chlorides (2.4.4)
Maximum 360 ppm.
To 0.46 g add 10 mL of dilute nitric acid R and 30 mL of water R. Heat on a water-bath for 5 min. Cool, dilute to 50 mL with water R, mix well and filter.
Sulfates (2.4.13)
Maximum 0.1 per cent.
To 0.50 g add 2.5 mL of dilute nitric acid R and dilute to 50 mL with distilled water R. Heat on a water-bath for 5 min, shake for 2 min, cool and filter.
Iron (2.4.9)
Maximum 75 ppm.
Ignite 0.66 g at 800 ± 50 °C for 2 h. Dissolve the residue in 2.5 mL of dilute hydrochloric acid R with gentle heating for 10 min. Cool and dilute to 50 mL with water R.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in vacuo at 60 °C for 3 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
To 0.450 g add 10 mL of acetic anhydride R and 50 mL of glacial acetic acid R, heat at 50 °C and stir for 10 min. Allow to cool (a clear solution is not obtained). Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20). Carry out a blank titration.
1 mL of 0.1 M perchloric acid is equivalent to 59.47 mg of C34H30N2O6S.
STORAGE
Protected from light.
IMPURITIES
Specified impurities C, D.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B.

A. 1-methyl-2-[(Z)-2-(thiophen-2-yl)eth-1-en-1-yl]-1,4,5,6-tetrahydropyrimidine,
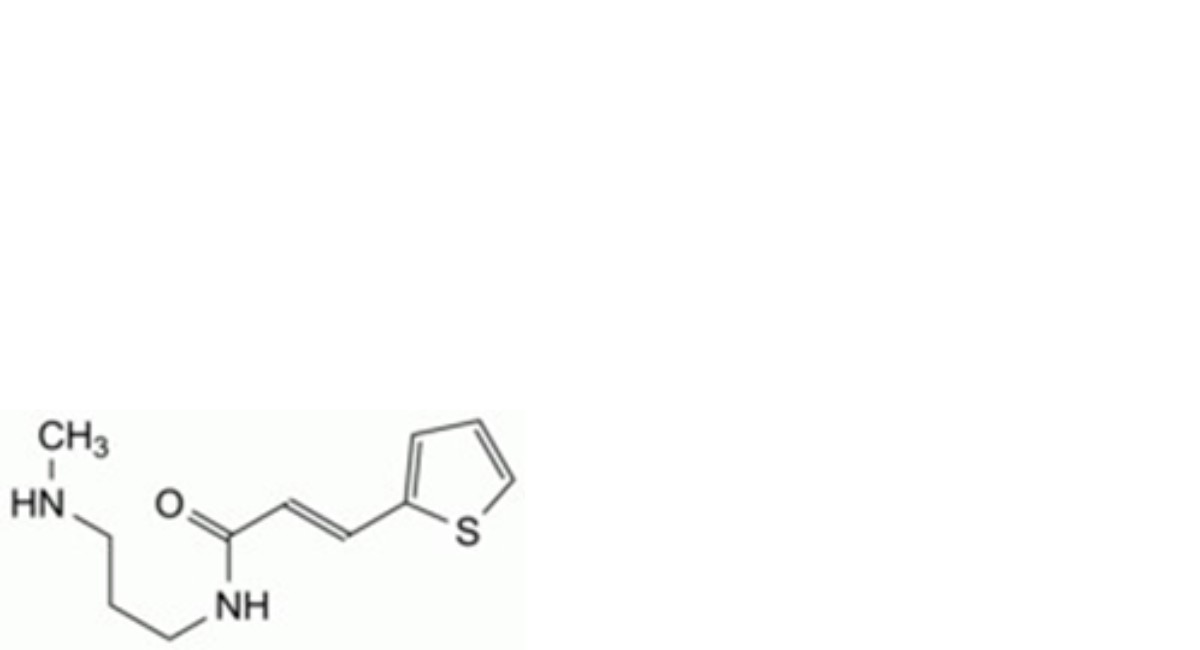
B. (E)-N-[3-(methylamino)propyl]-3-(thiophen-2-yl)prop-2-enamide,

C. thiophene-2-carbaldehyde,

D. 1,2-dimethyl-1,4,5,6-tetrahydropyrimidine.