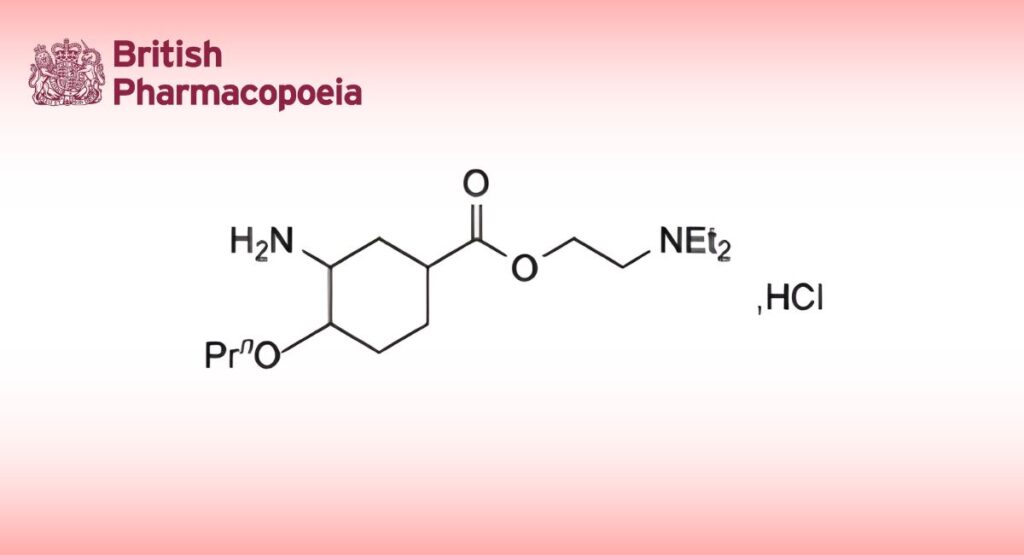
C16H26N2O3HCl 330.9 5875-06-9
Action and use
Local anaesthetic.
Preparation
Proxymetacaine Eye Drops
DEFINITION
Proxymetacaine Hydrochloride is 2-diethylaminoethyl 3-amino-4-propoxybenzoate hydrochloride. It contains not less than 98.0% and not more than 102.0% of C16H26N2O3,HCl, calculated with reference to the dried substance.
CHARACTERISTICS
A white or almost white, crystalline powder.
Soluble in water; very soluble in absolute ethanol; practically insoluble in ether.
IDENTIFICATION
A. The light absorption, Appendix II B, in the range 220 to 350 nm of a 0.002% w/v solution exhibits three maxima, at 231, 268 and 310 nm. The absorbances at the maxima at 268 nm and at 310 nm are about 0.58 and about 0.32, respectively.
B. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of proxymetacaine hydrochloride (RS 303).
C. A 5% w/v solution yields the reaction characteristic of primary aromatic amines and the reactions characteristic of chlorides, Appendix VI.
TESTS
Acidity
pH of a 1% w/v solution, 5.7 to 6.4, Appendix V L.
Related substances
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions of the substance being examined in methanol.
(1) 2.0% w/v of the substance being examined.
(2) 0.020% w/v of the substance being examined.
(3) 0.010% w/v of the substance being examined.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel GF254.
(b) Use the mobile phase as described below.
(c) Apply 10 μL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air, heat at 105° for 10 minutes, allow to cool and examine under ultraviolet light (254 nm).
MOBILE PHASE
5 volumes of diethylamine, 30 volumes of ethyl acetate and 75 volumes of toluene.
LIMITS
Any secondary spot in the chromatogram obtained with solution (1);
is not more intense than the spot in the chromatogram obtained with solution (2) (1%);
not more than one such spot is more intense than the spot in the chromatogram obtained with solution (3) (0.5%).
Disregard any spot remaining on the line of application.
B. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in methanol.
(1) 2.0% w/v of the substance being examined.
(2) 0.0050% w/v of 3-amino-4-propoxybenzoic acid BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel GF254.
(b) Use the mobile phase as described below.
(c) Apply 10 μL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air, examine under ultraviolet light (254 nm).
MOBILE PHASE
4 volumes of glacial acetic acid, 20 volumes of cyclohexane and 80 volumes of 1,4-dioxan.
LIMITS
Any secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2) (0.25%). The principal spot remains on or near the line of application.
Loss on drying
When dried at 105° for 3 hours, loses not more than 0.5% of its weight. Use 1 g.
Sulfated ash
Not more than 0.15%, Appendix IX A.
ASSAY
Carry out Method I for non-aqueous titration, Appendix VIII A, using 0.25 g, 20 mL of mercury(II) acetate solution and 1-naphtholbenzein solution as indicator. Each mL of 0.1M perchloric acid VS is equivalent to 16.54 mg of C16H26N2O3,HCl.
STORAGE
Proxymetacaine Hydrochloride should be protected from light.