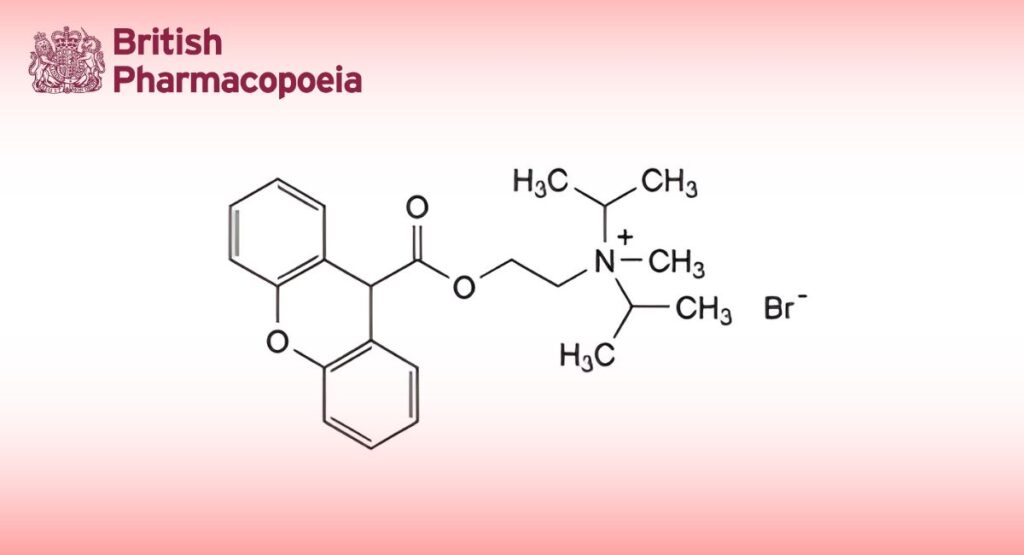
(Ph. Eur. monograph 0857)
C23H30BrNO3 448.4 50-34-0
Action and use
Anticholinergic.
Preparation
Propantheline Tablets
DEFINITION
N-Methyl-N,N-bis(1-methylethyl)-2-[(9H-xanthen-9-ylcarbonyl)oxy]ethanaminium bromide.
Content
98.5 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or yellowish-white, slightly hygroscopic powder.
Solubility
Very soluble in water, in ethanol (96 per cent) and in methylene chloride.
IDENTIFICATION
A. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution: Dissolve 60 mg in methanol R and dilute to 100.0 mL with the same solvent. Dilute 10.0 mL of this solution to 100.0 mL with methanol R.
Spectral range: 230-350 nm.
Absorption maxima: At 246 nm and 282 nm.
Specific absorbance at the absorption maxima:
— at 246 nm: 115 to 125;
— at 282 nm: 57 to 63.
B. Dissolve 0.2 g in 15 mL of water R and add 1 mL of strong sodium hydroxide solution R. Boil for 2 min and cool slightly. Add 7.5 mL of dilute hydrochloric acid R and filter. Wash the residue with water R and recrystallise from ethanol (50 per cent V/V) R. Dry at 100-105 °C for 1 h. Dissolve about 10 mg of the residue in 5 mL of sulfuric acid R. The solution has an intense yellow colour and shows an intense yellowish-green fluorescence when examined in ultraviolet light at 365 nm.
C. Dissolve 50 mg in 0.1 mL of water R in a 25 mL flask and add 1 mL of a saturated solution of potassium permanganate R. Attach a fractionating column and a condenser, with the end of the delivery tube immersed in 1 mL of water R in a test-tube placed in a bath of iced water. Distil fairly vigorously and continue heating for 1 min after a dry residue has been obtained in the flask. Prepare a blank by introducing into an identical test-tube a volume of water R equal to that of the distillate. Place the tubes in a bath of iced water. To each tube, add 0.5 mL of a 20 per cent V/V solution of morpholine R and 0.5 mL of a freshly prepared 50 g/L solution of sodium nitroprusside R. Mix and allow to stand at 0 °C for 5 min, and then at room temperature for 3 min. No blue colour develops in either tube. Add 1 g of ammonium sulfate R, mix and allow to stand for 15 min. A stable, intense pink colour develops in the test solution. A brownish-yellow colour develops in the blank.
D. It gives reaction (a) of bromides (2.3.1).
TESTS
Appearance of solution
The solution is clear (2.2.1).
Dissolve 0.6 g in water R and dilute to 20 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Solvent mixture: acetonitrile R, water R (40:60 V/V).
Test solution (a): Dissolve 6 mg of the substance to be examined in the solvent mixture and dilute to 50 mL with the solvent mixture.
Test solution (b): Dissolve 6 mg of the substance to be examined in 30 mL of the solvent mixture. Add 5 mL of reference solution (b) and dilute to 50 mL with the solvent mixture.
Test solution (c): Dissolve 6 mg of xanthydrol R1 and 6 mg of the substance to be examined in the solvent mixture, then dilute to 50 mL with the solvent mixture.
Reference solution (a): Dissolve 6 mg of xanthydrol R1 in the solvent mixture and dilute to 50 mL with the solvent mixture.
Reference solution (b): Dilute 5 mL of reference solution (a) to 50 mL with the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: Mixture of equal volumes of acetonitrile R and of a solution containing 28 g/L of sodium perchlorate R and 11 g/L of phosphoric acid R, adjusted to pH 3.8 with strong sodium hydroxide solution R and then with 0.1 M sodium hydroxide.
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 206 nm.
Injection: 20 μL of test solutions (a), (b), (c) and reference solution (a).
Run time: Twice the retention time of propantheline.
System suitability: Test solution (c):
— in the chromatogram obtained with test solution (a), there is no peak corresponding to the principal peak in the chromatogram obtained with reference solution (a);
— resolution: minimum 8.0 between the peaks due to propantheline and xanthydrol.
Limits Test solution (b):
— any impurity: for each impurity, not more than the area of the peak due to xanthydrol (1.0 per cent), and not more than one such peak has an area greater than or equal to 0.5 times the area of the peak due to xanthydrol (0.5 per
cent);
— disregard limit: disregard any peak with a retention time relative to propantheline of less than 0.2 (bromide); disregard the peak due to xanthydrol.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.400 g in 50 mL of acetic anhydride R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid corresponds to 44.84 mg of C23H30BrNO3.
STORAGE
In an airtight container.