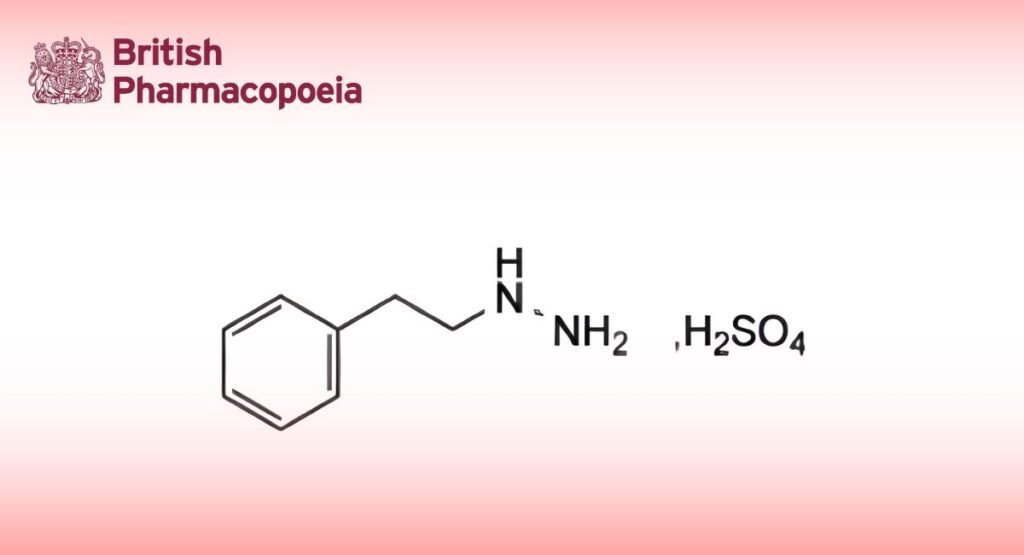
Phenelzine Sulphate
234.3 156-51-4
Action and use
Monoamine oxidase inhibitor; antidepressant.
Preparation
Phenelzine Tablets
DEFINITION
Phenelzine Sulfate is phenethylhydrazine hydrogen sulfate. It contains not less than 98.0% and not more than 102.0% of C8H12N2, H2SO4, calculated with reference to the dried substance.
CHARACTERISTICS
A white powder or pearly platelets.
Freely soluble in water; practically insoluble in ethanol (96%) and in ether.
IDENTIFICATION
A. The light absorption, Appendix II B, in the range 230 to 350 nm of a 0.1% w/v solution in 0.05M sulfuric acid exhibits three well-defined maxima, at 252, 258 and 263 nm. The absorbances at the maxima are about 0.62, about 0.77 and about 0.58, respectively.
B. Dissolve 0.1 g in 5 mL of water, make alkaline with 5M sodium hydroxide and add 1 mL of cupri-tartaric solution R1. A red precipitate is produced.
C. Yields reaction A characteristic of sulfates, Appendix VI.
TESTS
Melting point
164° to 168°, Appendix V A.
Loss on drying
When dried over phosphorus pentoxide at a pressure not exceeding 0.7 kPa for 24 hours, loses not more than 1.0% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) 0.026% w/v of the substance to be examined in the mobile phase.
(2) 0.026% w/v phenelzine sulfate BPCRS in the mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) A stainless steel column (15 cm × 3.9 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Waters
Symmetry C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 210 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
40 volumes of methanol R2 and 60 volumes of a solution of 0.216% w/v sodium octanesulfonate and 0.68% w/v potassium dihydrogen phosphate previously adjusted to pH 3.0 with orthophosphoric acid.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2), the symmetry factor of the principal peak is between 0.8 and 2.0.
DETERMINATION OF CONTENT
Calculate the content of C8H12N2, H2SO4 in the substance being examined using the declared content of C8H12N2, H2SO4 in phenelzine sulfate BPCRS.
STORAGE
Phenelzine Sulfate should be protected from light.