(Ph. Eur. monograph 0421)
C11H12N2O 188.2 60-80-0
Action and use
Analgesic; used to test hepatic drug-metabolizing activity.
DEFINITION
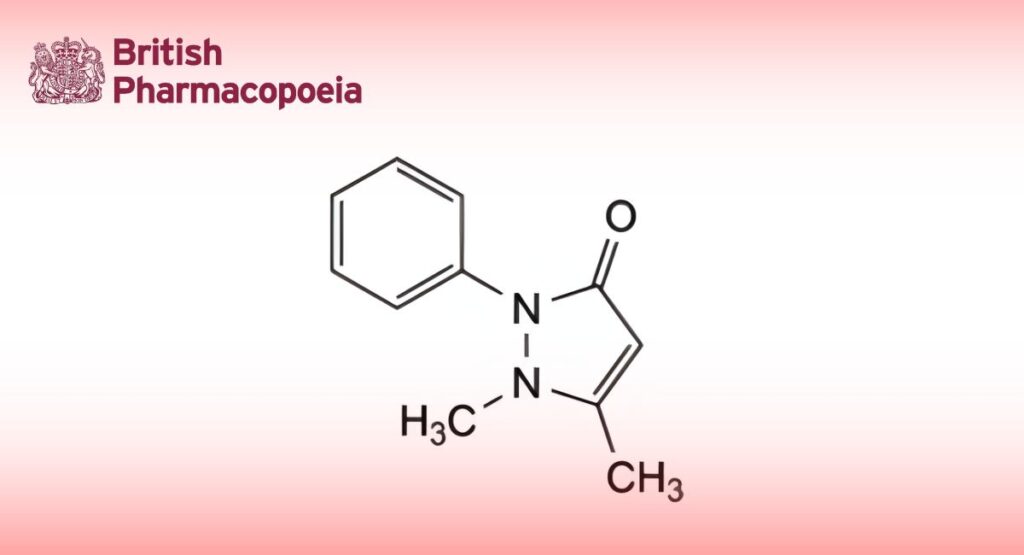
1,5-Dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or colourless crystals.
Solubility
Very soluble in water, in ethanol (96 per cent) and in methylene chloride.
IDENTIFICATION
First identification: A, B.
Second identification: A, C, D.
A. Melting point (2.2.14): 109 °C to 113 °C.
B. Infrared absorption spectrophotometry (2.2.24).Comparison phenazone CRS.
C. To 1 mL of solution S (see Tests) add 4 mL of water R and 0.25 mL of dilute sulfuric acid R. Add 1 mL of sodium nitrite solution R; a green colour develops.
D. To 1 mL of solution S add 4 mL of water R and 0.5 mL of ferric chloride solution R2. A red colour develops which is discharged on the addition of dilute sulfuric acid R.
TESTS
Solution S
Dissolve 2.5 g in carbon dioxide-free water R and dilute to 50 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
To 10 mL of solution S add 0.1 mL of phenolphthalein solution R; the solution is colourless. Add 0.2 mL of 0.01 M sodium hydroxide; the solution is red. Add 0.25 mL of methyl red solution R and 0.4 mL of 0.01 M hydrochloric acid; the solution is red or yellowish-red.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 10.0 mL with the mobile phase.
Reference solution (b): Dissolve 5 mg of phenazone impurity A CRS in the mobile phase, add 10 mL of the test solution and dilute to 20.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 50.0 mL with the mobile phase.
Reference solution (c): Dissolve 5.0 mg of phenazone impurity A CRS in the mobile phase and dilute to 20.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 10.0 mL with the mobile phase.
Column:
— size: l = 0.15 m, Ø = 6.0 mm;
— stationary phase: spherical octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: Dissolve 6.8 g of potassium dihydrogen phosphate R in water R and dilute to 1000 mL with the same solvent. Add 2 mL of triethylamine R and adjust to pH 7.0 with sodium hydroxide solution R. Add 430 mL of methanol R.
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 10 μL.
Run time: 3 times the retention time of phenazone.
Relative retention: With reference to phenazone (retention time = about 13 min): impurity A = about 0.8.
System suitability: Reference solution (b):
— resolution: minimum 3.0 between the peaks due to impurity A and phenazone.
Limits:
— impurity A: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (c) (0.05 per cent);
— unspecified impurities: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent);
— total: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent);
— disregard limit: 0.3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.03 per cent).
Chlorides (2.4.4)
Maximum 100 ppm.
Dilute 10 mL of solution S to 15 mL with water R.
Sulfates (2.4.13)
Maximum 100 ppm.
Dissolve 1.5 g in distilled water R and dilute to 15 mL with the same solvent.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in vacuo at 60 °C for 6 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.150 g in 20 mL of water R. Add 2 g of sodium acetate R, 1 mL of dilute acetic acid R and 25.0 mL of 0.05 M iodine. Allow to stand protected from light for 30 min. Add 25 mL of methylene chloride R and shake until the precipitate dissolves. Titrate with 0.1 M sodium thiosulfate, using 1 mL of starch solution R, added towards the end of the titration, as indicator. Carry out a blank titration.
1 mL of 0.05 M iodine is equivalent to 9.41 mg of C11H12N2O.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A.
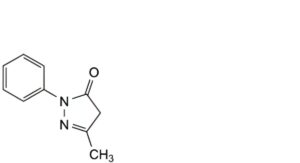
A. 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one.