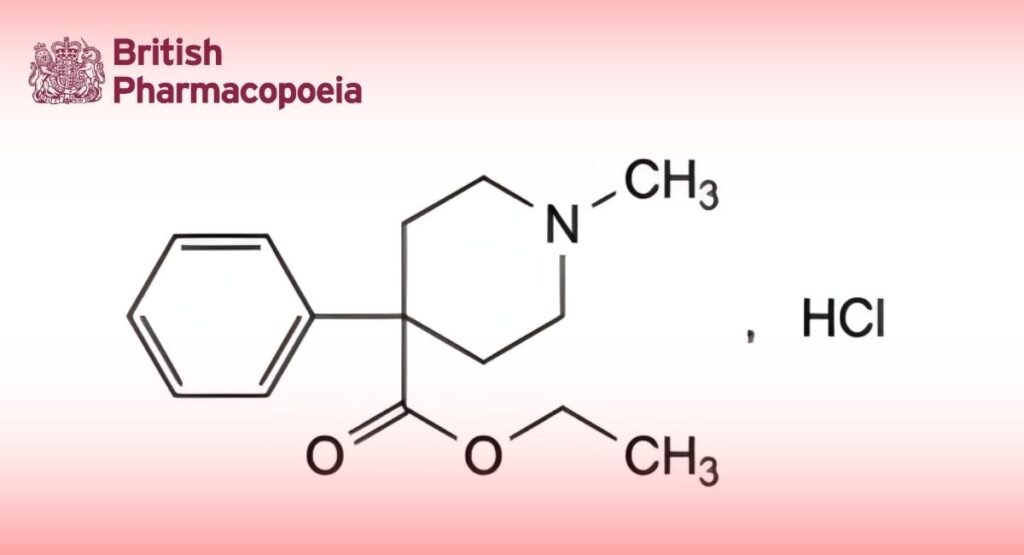
(Ph. Eur. monograph 0420)
C15H22ClNO2 283.8 50-13-5
Action and use
Opioid receptor agonist; analgesic.
Preparations
Pethidine Injection
Pethidine Tablets
DEFINITION
Ethyl 1-methyl-4-phenylpiperidine-4-carboxylate hydrochloride.
Content
99.0 per cent to 101.0 per cent (dried substance).
PRODUCTION
If intended for use in the manufacture of parenteral preparations, the manufacturing process is validated to show that the content of impurity B is not more than 0.1 ppm.
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Very soluble in water, freely soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: B, D.
Second identification: A, C, D.
A. Melting point (2.2.14): 187 °C to 190 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: Ph. Eur. reference spectrum of pethidine hydrochloride.
C. Dissolve 0.1 g in 10 mL of ethanol R and add 10 mL of picric acid solution R. A crystalline precipitate is formed which, when washed with water R and dried at 100-105 °C, melts (2.2.14) at 186 °C to 193 °C. Mix equal quantities of the precipitate and the substance to be examined and determine the melting point of the mixture. The melting point is at least 20 °C lower than that of the precipitate.
D. To 5 mL of solution S (see Tests) add 5 mL of water R. The solution gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dissolve 0.5 g in carbon dioxide-free water R and dilute to 25 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
To 10 mL of solution S add 0.2 mL of methyl red solution R and 0.2 mL of 0.01 M sodium hydroxide. The solution is yellow.
Add 0.3 mL of 0.01 M hydrochloric acid. The solution is red.
Impurity B
Liquid chromatography (2.2.29).
Test solution (a): Dissolve 0.100 g of the substance to be examined in a mixture of 20 volumes of acetonitrile R and 80 volumes of water R and dilute to 25.0 mL with the same mixture of solvents.
Test solution (b): Dissolve 0.125 g of the substance to be examined in a mixture of 20 volumes of acetonitrile R and 80 volumes of water R and dilute to 10.0 mL with the same mixture of solvents.
Reference solution (a): Dilute 0.5 mL of test solution (a) to 100.0 mL with a mixture of 20 volumes of acetonitrile R and 80 volumes of water R.
Reference solution (b): Dissolve 10.0 mg of pethidine impurity A CRS in a mixture of 20 volumes of acetonitrile R and 80 volumes of water R and dilute to 100.0 mL with the same mixture of solvents.
Reference solution (c): Dissolve 12.5 mg of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine R in a mixture of 20 volumes of acetonitrile R and 80 volumes of water R and dilute to 10.0 mL with the same mixture of solvents. Dilute 1.0 mL of the solution to 100.0 mL with a mixture of 20 volumes of acetonitrile R and 80 volumes of water R.
Reference solution (d): Dilute 5.0 mL of reference solution (b) and 1.0 mL of reference solution (c) to 100.0 mL with a mixture of 20 volumes of acetonitrile R and 80 volumes of water R.
Column:
— size: l = 0.25 m, Ø = 4.0 mm,
— stationary phase: spherical end-capped octadecylsilyl silica gel for chromatography R (5 μm) with a specific surface area of 340 m2 /g, a pore size of 10 nm and a carbon loading of 19 per cent.
Mobile phase:
— mobile phase A: mix equal volumes of a 42.0 g/L solution of sodium perchlorate R and of a 11.6 g/L solution of phosphoric acid R, adjust to pH 2.0 with triethylamine R,
— mobile phase B: acetonitrile R,
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 15 | 80 → 75 | 20 → 25 |
| 15 – 31 | 75 → 55 | 25 → 45 |
| 31 – 40 | 55 | 45 |
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 210 nm.
Injection: 50 μL; inject test solution (b) and reference solution (d).
Relative retention: With reference to pethidine (retention time = about 24 min): impurity B = about 0.66; impurity A = about 0.68.
System suitability: Reference solution (d):
— signal-to-noise ratio: minimum 10 for the first peak,
— peak-to-valley ratio: minimum 4, where Hp = height above the baseline of the peak due to impurity B, and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity A.
Limit:
— impurity B: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (d) (10 ppm) if intended for non-parenteral administration.
Related substances
Liquid chromatography (2.2.29) as described in the test for impurity B with the following modifications.
Injection: 20 μL; inject test solution (a) and reference solution (a).
Limits:
— any impurity: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent),
— total: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (1.0 per cent),
— disregard limit: 0.1 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.220 g in 50 mL of alcohol R. Add 5.0 mL of 0.01 M hydrochloric acid. Titrate with 0.1 M sodium hydroxide determining the end-point potentiometrically (2.2.20). Read the volume added between the 2 points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 28.38 mg of C15H22ClNO2.
STORAGE
In an airtight container, protected from light.
LABELLING
The label states, where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
IMPURITIES
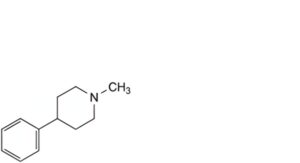
A. 1-methyl-4-phenylpiperidine (MPP),
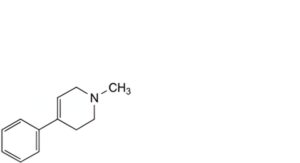
B. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP),
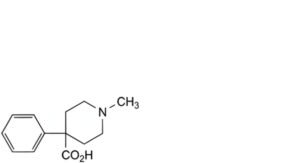
C. 1-methyl-4-phenylpiperidine-4-carboxylic acid,
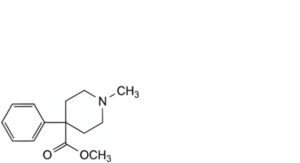
D. methyl 1-methyl-4-phenylpiperidine-4-carboxylate,
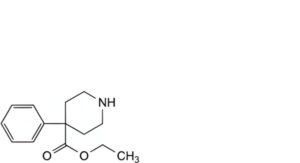
E. ethyl 4-phenylpiperidine-4-carboxylate,
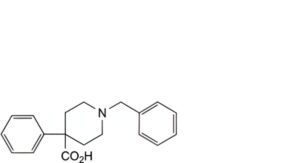
F. 1-benzyl-4-phenylpiperidine-4-carboxylic acid,
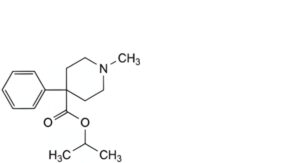
G. 1-methylethyl 1-methyl-4-phenylpiperidine-4-carboxylate,
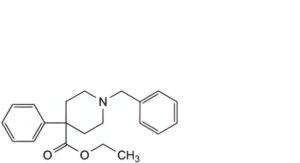
H. ethyl 1-benzyl-4-phenylpiperidine-4-carboxylate,
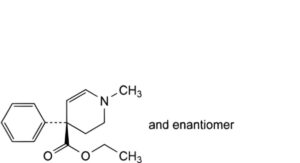
I. ethyl (4RS)-1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-4-carboxylate,
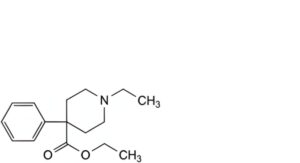
J. ethyl 1-ethyl-4-phenylpiperidine-4-carboxylate.