(Ph. Eur. monograph 2000)
C22H33NO4 375.5 17146-95-1
Action and use
Opioid receptor agonist; analgesic.
Preparation
Pentazocine Injection
DEFINITION
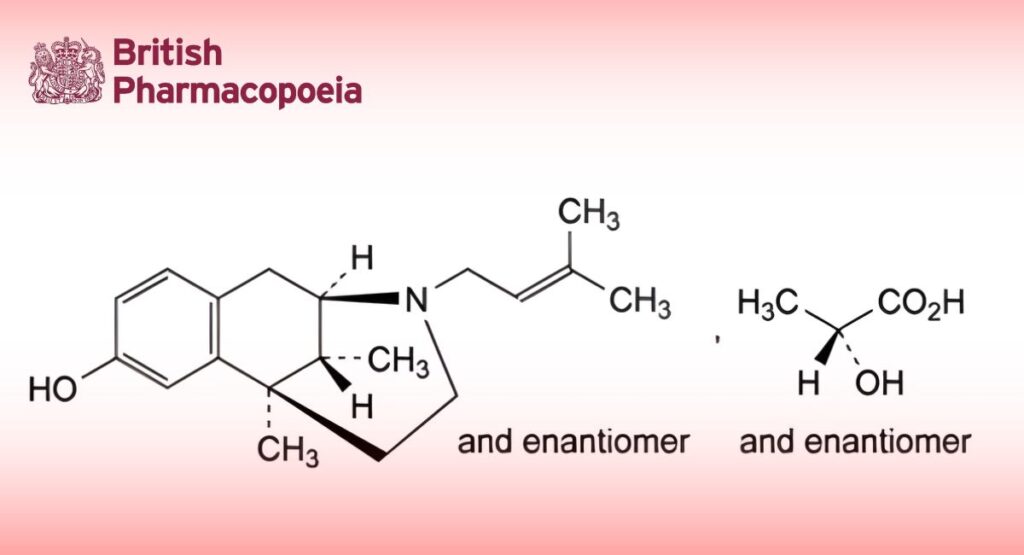
(2RS,6RS,11RS)-6,11-Dimethyl-3-(3-methylbut-2-enyl)-1,2,3,4,5,6-hexahydro-2,6-methano-3-benzazocin-8-ol (2RS)-2- hydroxypropanoate.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Sparingly soluble in water, freely soluble in methanol, slightly soluble in methylene chloride.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison: Ph. Eur. reference spectrum of pentazocine lactate.
TESTS
pH (2.2.3)
5.5 to 6.5.
Dissolve 0.1 g in 10 mL of carbon dioxide-free water R.
Absorbance (2.2.25)
0.50 to 0.54, determined at the absorption maximum at 278 nm.
Dissolve 0.10 g in 10.0 mL of 1 M hydrochloric acid and dilute to 100.0 mL with water R. Dilute 10.0 mL of the solution to 100.0 mL with water R.
Related substances
Thin-layer chromatography (2.2.27).
Test solution: Dissolve 0.20 g of the substance to be examined in methylene chloride R and dilute to 10 mL with the same solvent.
Reference solution (a): Dissolve 100 mg of the substance to be examined in acetic anhydride R and dilute to 5 mL with the same solvent. Heat at 80 °C for 10 min. Dilute 1 mL of the solution to 10 mL with methanol R. Mix 1 mL of this solution with 1 mL of the test solution.
Reference solution (b): Dilute 1 mL of the test solution to 100 mL with methylene chloride R. Dilute 2 mL of this solution to 10 mL with methylene chloride R.
Plate: TLC silica gel F254 plate R.
Mobile phase: isopropylamine R, methanol R, methylene chloride R (3:3:94 V/V/V).
Application: 10 μL.
Development: Over 2/3 of the plate.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm. Heat at 100-105 °C for 15 min. Allow to cool. Expose to iodine vapour and re-examine in ultraviolet light at 254 nm.
System suitability: Reference solution (a):
— the chromatogram shows 2 clearly separated spots.
Limits: By each method of detection:
— any impurity: any spots, apart from the principal spot, are not more intense than the spot in the chromatogram obtained with reference solution (b) (0.2 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.300 g in 30 mL of anhydrous acetic acid R and add 30 mL of dioxan R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 37.55 mg of C22H33NO4.
STORAGE
Protected from light.