(Ph. Eur. monograph 1463)
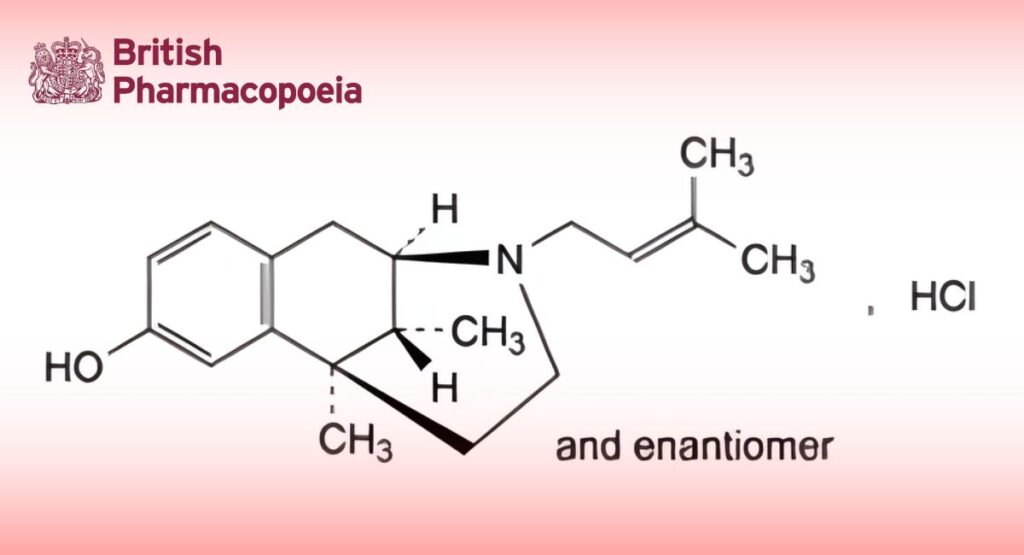
C19H28ClNO 321.9 64024-15-3
Action and use
Opioid receptor agonist; analgesic.
Preparations
Pentazocine Capsules
Pentazocine Tablets
DEFINITION
Pentazocine hydrochloride contains not less than 99.0 per cent and not more than the equivalent of 101.0 per cent of (2RS,6RS,11RS)-6,11-dimethyl-3-(3-methylbut-2-enyl)-1,2,3,4,5,6-hexahydro-2,6-methano-3-benzazocin-8-ol hydrochloride, calculated with reference to the dried substance.
CHARACTERS
A white or almost white powder, sparingly soluble in water, soluble in ethanol (96 per cent) and sparingly soluble in methylene chloride.
It shows polymorphism (5.9).
IDENTIFICATION
A. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the Ph. Eur. reference spectrum of pentazocine hydrochloride.
B. It gives reaction (a) of chlorides (2.3.1).
TESTS
pH (2.2.3)
Dissolve 0.1 g in 10 mL of carbon dioxide-free water R. The pH of the solution is 4.0 to 6.0.
Absorbance (2.2.25)
Dissolve 0.100 g in a mixture of 20 mL of water R and 1 mL of 1 M hydrochloric acid, and dilute to 100.0 mL with water R.
To 10.0 mL add 1 mL of 1 M hydrochloric acid and dilute to 100.0 mL with water R. The absorbance at the absorption maximum at 278 nm is 0.59 to 0.63, calculated with reference to the dried substance.
Related substances
Examine by thin-layer chromatography (2.2.27), using a TLC silica gel F254 plate R.
Test solution: Dissolve 0.20 g in 3 mL of methanol R and dilute to 10 mL with methylene chloride R.
Reference solution (a): Dilute 1 mL of the test solution to 100 mL with methylene chloride R.
Reference solution (b): Dilute 5 mL of reference solution (a) to 10 mL with methylene chloride R.
Reference solution (c): Dilute 5 mL of reference solution (a) to 20 mL with methylene chloride R.
Apply to the plate 10 μL of each solution. Develop over a path corresponding to two-thirds of the plate height using a mixture of 3 volumes of isopropylamine R, 3 volumes of methanol R and 94 volumes of methylene chloride R. Allow the plate to dry in air and examine in ultraviolet light at 254 nm. Heat the plate at 100-105 °C for 15 min, allow to cool, expose to iodine vapour and re-examine under ultraviolet light at 254 nm. By each method of visualisation: any spot in the
chromatogram obtained with the test solution, apart from the principal spot, is not more intense than the spot obtained with reference solution (a) (1 per cent); not more than 1 such spot is more intense than the spot in the chromatogram obtained with reference solution (b) (0.5 per cent); and not more than 4 such spots are more intense than the spot in the chromatogram obtained with reference solution (c) (0.25 per cent).
Loss on drying (2.2.32)
Not more than 0.5 per cent, determined on 1.000 g by drying at 60 °C at a pressure not exceeding 0.7 kPa for 4 h.
Sulfated ash (2.4.14)
Not more than 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.250 g in 50 mL of ethanol (96 per cent) R. Add 5 mL of 0.01 M hydrochloric acid. Carry out a potentiometric titration (2.2.20), using 0.1 M sodium hydroxide. Read the volume added between the 2 points of inflection.
1 mL of 0.1 M sodium hydroxide is equivalent to 32.19 mg of C19H28ClNO.
STORAGE
Store protected from light.