(Ph. Eur. monograph 1252)
Action and use
Low molecular weight heparin.
DEFINITION
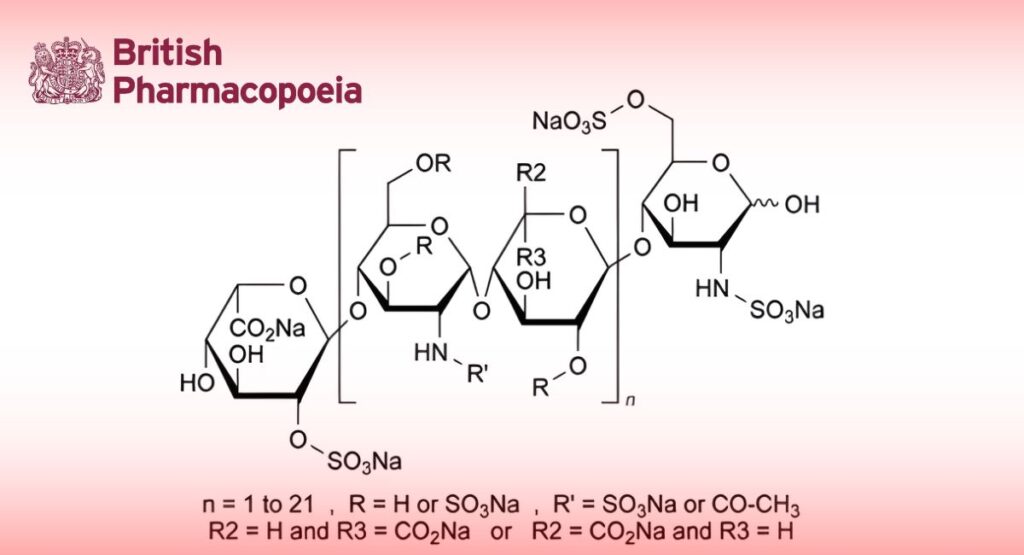
Sodium salt of a low-molecular-mass heparin that is obtained by radical-catalysed depolymerisation, with hydrogen peroxide and with a cupric salt, of heparin from porcine intestinal mucosa. The majority of the components have a 2-O-sulfo-α-L-idopyranuronic acid structure at the non-reducing end and a 2-N,6-O-disulfo-D-glucosamine structure at the reducing end of their chain.
Parnaparin sodium complies with the monograph Low-molecular-mass heparins (0828), with the modifications and additional requirements below.
The mass-average relative molecular mass ranges between 4000 and 6000 with a characteristic value of about 5000.
The degree of sulfatation is 2.0 to 2.6 per disaccharide unit.
The potency is not less than 75 IU and not more than 110 IU of anti-factor Xa activity per milligram calculated with reference to the dried substance. The ratio of anti-factor Xa activity to anti-factor IIa activity is between 1.5 and 3.0.
IDENTIFICATION
Carry out identification test A as described in the monograph Low-molecular-mass heparins (0828) using parnaparin sodium CRS.
Carry out identification test C as described in the monograph Low-molecular-mass heparins (0828). In order to verify the suitability of the system in the lower molecular mass ranges (for example Mr 2000), a suitable reference preparation is used. The following requirements apply.
The mass-average relative molecular mass ranges between 4000 and 6000. The mass percentage of chains lower than 3000 is not more than 30 per cent. The mass percentage of chains between 3000 and 8000 ranges between 50 per cent and 60 per cent.
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution Y5 (2.2.2, Method II).
Dissolve 1.5 g in 10 mL of water R.