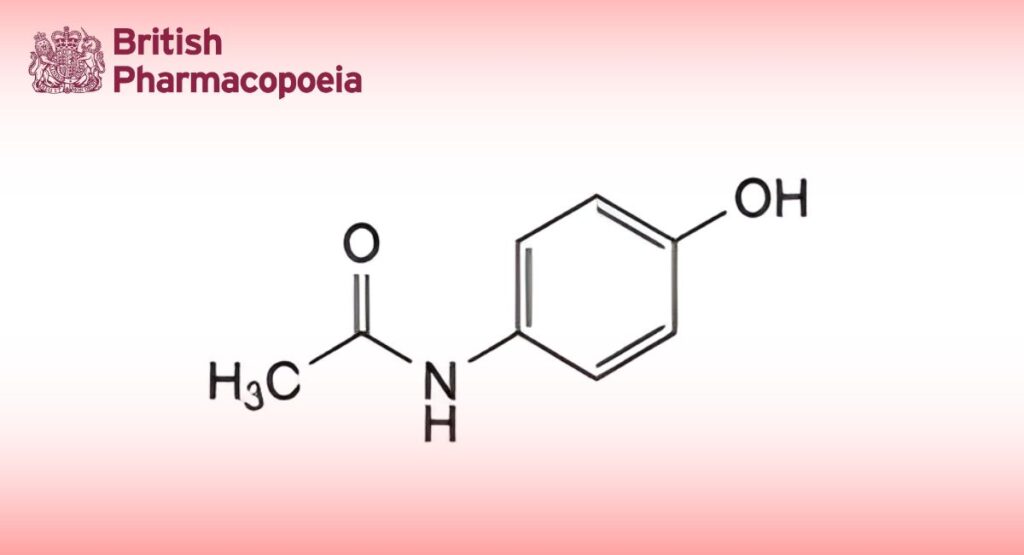
(Ph. Eur. monograph 0049)
C8H9NO2 151.2 103-90-2
Action and use
Analgesic; antipyretic.
Preparations
Co-codamol Tablets
Co-codamol Capsules
Co-codamol Effervescent Tablets
Paracetamol Effervescent Tablets
Co-dydramol Tablets
Co-proxamol Tablets
Paracetamol Capsules
Paracetamol Infusion
Paediatric Paracetamol Oral Solution
Paediatric Paracetamol Oral Suspension
Paracetamol Oral Solution
Paracetamol Oral Suspension
Paracetamol Suppositories
Paracetamol Tablets
Paracetamol and Caffeine Tablets
Paracetamol and Caffeine Soluble Tablets
Paracetamol Dispersible Tablets
Paracetamol Soluble Tablets
Paracetamol, Codeine Phosphate and Caffeine Capsules
Paracetamol, Codeine Phosphate and Caffeine Tablets
DEFINITION
N-(4-Hydroxyphenyl)acetamide.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Sparingly soluble in water, freely soluble in ethanol (96 per cent), very slightly soluble in methylene chloride.
IDENTIFICATION
First identification: B.
Second identification: A.
A. Melting point (2.2.14).
Determination A: Determine the melting point of the substance to be examined.
Result A: 168 °C to 172 °C.
Determination B: Mix equal parts of the substance to be examined and paracetamol CRS and determine the melting point of the mixture.
Result B: The absolute difference between the melting point of the mixture and the value obtained in determination A is not greater than 2 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: paracetamol CRS.
TESTS
Related substances
Liquid chromatography (2.2.29).
Solvent mixture: methanol R, water R (15:85 V/V).
Test solution: Dissolve 50.0 mg of the substance to be examined in 0.75 mL of methanol R and dilute to 5.0 mL with water R.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 20.0 mL with the solvent mixture.
Reference solution (b): Dissolve 5.0 mg of paracetamol impurity J CRS in 25 mL of methanol R and dilute to 250.0 mL with the solvent mixture. Dilute 1.0 mL of the solution to 200.0 mL with the solvent mixture.
Reference solution (c): Dissolve 5.0 mg of paracetamol impurity K CRS in the solvent mixture and dilute to 100.0 mL with the solvent mixture. Dilute 1.0 mL of the solution to 10.0 mL with the solvent mixture.
Reference solution (d): Dilute 1.0 mL of reference solution (c) to 10.0 mL with the solvent mixture.
Reference solution (e): Mix 1 mL of reference solution (a) and 1 mL of reference solution (c) and dilute to 10 mL with the solvent mixture.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped solid core octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase:
— mobile phase A: dissolve 1.7 g of potassium dihydrogen phosphate R and 1.8 g of dipotassium hydrogen phosphate R in water for chromatography R and dilute to 1000 mL with the same solvent;
— mobile phase B: methanol R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 1.5 | 95 | 5 |
| 1.5 – 14.4 | 95 → 90 | 5 → 10 |
| 14.4 – 28.8 | 90 | 10 |
| 28.8 – 57.6 | 90 → 66 | 10 → 34 |
| 57.6 – 60 | 66 | 34 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 254 nm.
Autosampler: Set at 5 °C.
Injection: 50 μL of the test solution and reference solutions (a), (b), (d) and (e).
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peak due to impurity J; use the chromatogram obtained with reference solution (d) to identify the peak due to impurity K.
Relative retention: With reference to paracetamol (retention time = about 4 min): impurity K = about 0.4; impurity J = about 10.1.
System suitability: Reference solution (e):
— resolution: minimum 5.0 between the peaks due to impurity K and paracetamol.
Calculation of contents:
— for impurity J, use the concentration of impurity J in reference solution (b);
— for impurity K, use the concentration of impurity K in reference solution (d);
— for impurities other than J and K, use the concentration of paracetamol in reference solution (a).
Limits:
— impurity K: maximum 50 ppm;
— impurity J: maximum 10 ppm;
— unspecified impurities: for each impurity, maximum 0.05 per cent;
— total: maximum 0.2 per cent;
— reporting threshold: 0.03 per cent, except for impurities J and K.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.300 g in a mixture of 10 mL of water R and 30 mL of dilute sulfuric acid R. Boil under a reflux condenser for 1 h, cool and dilute to 100.0 mL with water R. To 20.0 mL of the solution add 40 mL of water R, 40 g of ice, 15 mL of dilute hydrochloric acid R and 0.1 mL of ferroin R. Titrate with 0.1 M cerium sulfate until a greenish-yellow colour is obtained.
Carry out a blank titration. 1 mL of 0.1 M cerium sulfate is equivalent to 7.56 mg of C8H9NO2.
STORAGE
Protected from light.
IMPURITIES
Specified impurities J, K.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B, C, D, E, F, G, H, I, L, M, N, O.
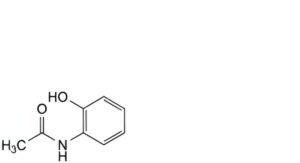
A. N-(2-hydroxyphenyl)acetamide,
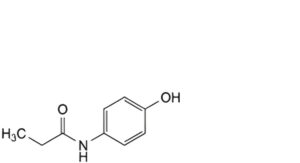
B. N-(4-hydroxyphenyl)propanamide,
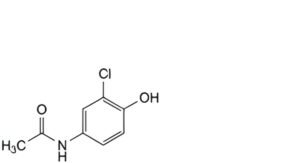
C. N-(3-chloro-4-hydroxyphenyl)acetamide,
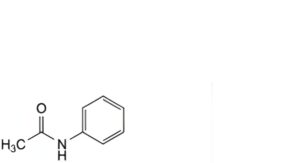
D. N-phenylacetamide,
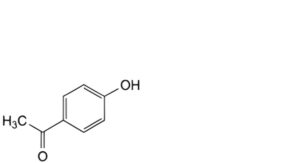
E. 1-(4-hydroxyphenyl)ethan-1-one,
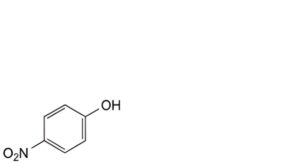
F. 4-nitrophenol,
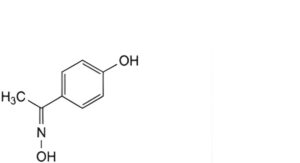
G. [1-(4-hydroxyphenyl)ethylidene]hydroxylamine,
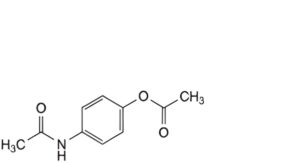
H. 4-acetamidophenyl acetate,
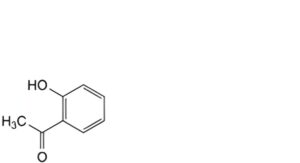
I. 1-(2-hydroxyphenyl)ethan-1-one,
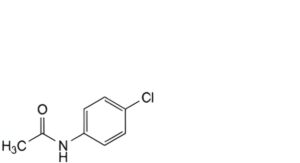
J. N-(4-chlorophenyl)acetamide (chloroacetanilide),
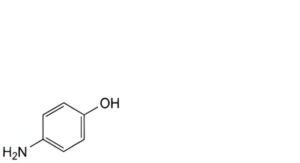
K. 4-aminophenol,
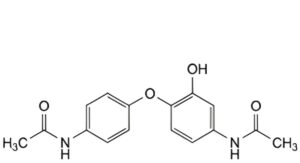
L. N-[4-(4-acetamido-2-hydroxyphenoxy)phenyl]acetamide,
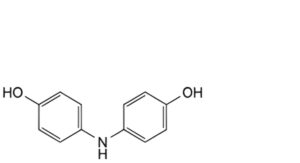
M. 4,4′-azanediyldiphenol,
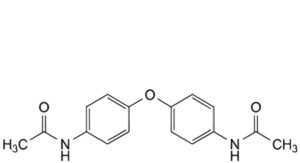
N. N,N′-[oxydi(4,1-phenylene)]diacetamide,
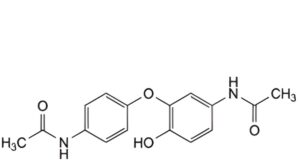
O. N-[4-(5-acetamido-2-hydroxyphenoxy)phenyl]acetamide.