General Notices
(Pancreas Powder, Ph. Eur. monograph 0350)
DEFINITION
Pancreas powder is prepared from the fresh or frozen pancreases of mammals. It contains various enzymes having proteolytic, lipolytic and amylolytic activities.
1 mg of pancreas powder contains not less than 1.0 Ph. Eur. U. of total proteolytic activity, 15 Ph. Eur. U. of lipolytic activity and 12 Ph. Eur. U. of amylolytic activity.
PRODUCTION
The animals from which pancreas powder is derived must fulfil the requirements for the health of animals suitable for human consumption.
CHARACTERS
Appearance
Slightly brown, amorphous powder.
Solubility
Partly soluble in water, practically insoluble in ethanol (96 per cent).
IDENTIFICATION
A. Triturate 0.5 g with 10 mL of water R and adjust to pH 8 with 0.1 M sodium hydroxide, using 0.1 mL of cresol red solution R as indicator. Divide the suspension into 2 equal parts (suspension (a) and suspension (b)). Boil suspension (a). To each suspension add 10 mg of fibrin congo red R, heat to 38-40 °C and maintain at this temperature for 1 h.
Suspension (a) is colourless or slightly pink and suspension (b) is distinctly more red.
B. Triturate 0.25 g with 10 mL of water R and adjust to pH 8 with 0.1 M sodium hydroxide, using 0.1 mL of cresol red solution R as indicator. Divide the suspension into 2 equal parts (suspension (a) and suspension (b)). Boil suspension (a). Dissolve 0.1 g of soluble starch R in 100 mL of boiling water R, boil for 2 min, cool and dilute to 150 mL with water R. To 75 mL of the starch solution add suspension (a) and to the remaining 75 mL add suspension (b). Heat each mixture to 38-40 °C and maintain at this temperature for 5 min.
To 1 mL of each mixture add 10 mL of iodine solution R2. The mixture obtained with suspension (a) has an intense blue- violet colour; the mixture obtained with suspension (b) has the colour of the iodine solution.
TESTS
Fat content
Maximum 5.0 per cent.
In an extraction apparatus, treat 1.0 g with light petroleum R1 for 3 h. Evaporate the solvent and dry the residue at 100- 105 °C for 2 h. The residue weighs a maximum of 50 mg.
Loss on drying (2.2.32)
Maximum 5.0 per cent, determined on 0.50 g by drying at 60 °C at a pressure not exceeding 670 Pa for 4 h.
Microbial contamination
TAMC: acceptance criterion 104 CFU/g (2.6.12).
TYMC: acceptance criterion 102 CFU/g (2.6.12).
Absence of Escherichia coli (2.6.13).
Absence of Salmonella (2.6.13).
ASSAY
Total proteolytic activity
The total proteolytic activity of pancreas powder is determined by comparing the quantity of peptides non-precipitable by a 50 g/L solution of trichloroacetic acid R released per minute from a substrate of casein solution with the quantity of such peptides released by pancreas powder (protease) BRP from the same substrate in the same conditions.
Casein solution: Suspend a quantity of casein BRP equivalent to 1.25 g of dried substance in 5 mL of water R, add 10 mL of 0.1 M sodium hydroxide and stir for 1 min. (Determine the water content of casein BRP prior to the test by heating at 60 °C in vacuo for 4 h.) Add 60 mL of water R and stir with a magnetic stirrer until the solution is practically clear. Adjust to pH 8.0 with 0.1 M sodium hydroxide or 0.1 M hydrochloric acid. Dilute to 100.0 mL with water R. Use the solution on the day of preparation.
Enterokinase solution: Dissolve 50 mg of enterokinase BRP in 0.02 M calcium chloride solution R and dilute to 50.0 mL with the same solvent. Use the solution on the day of preparation.
To avoid absorption of water formed by condensation, allow the preparation to be examined and the reference preparation to reach room temperature before opening the containers.
For the test suspension and the reference suspension, prepare the suspension and carry out the dilution at 0-4 °C.
Test suspension: Triturate 0.100 g of the substance to be examined for 5 min adding gradually 25 mL of 0.02 M calcium chloride solution R. Transfer completely to a volumetric flask and dilute to 100.0 mL with 0.02 M calcium chloride solution R. To 10.0 mL of this suspension add 10.0 mL of the enterokinase solution and heat on a water-bath at 35 ± 0.5 °C for 15 min. Cool and dilute with borate buffer solution pH 7.5 R at 5 ± 3 °C to a final concentration of about 0.065 Ph. Eur. U. of total proteolytic activity per millilitre calculated on the basis of the stated activity.
Reference suspension: Prepare a suspension of pancreas powder (protease) BRP as described for the test suspension but without the addition of enterokinase so as to obtain a known final concentration of about 0.065 Ph. Eur. U. per millilitre calculated on the basis of the stated activity.
Designate tubes in duplicate T, Tb, S1, S1b, S2, S2b, S3, S3b; designate a tube B.
Add borate buffer solution pH 7.5 R to the tubes as follows:
B: 3.0 mL;
S1 and S1b: 2.0 mL;
S2, S2b, T and Tb: 1.0 mL.
Add the reference suspension to the tubes as follows:
S1 and S1b: 1.0 mL;
S2 and S2b: 2.0 mL;
S3 and S3b: 3.0 mL.
Add 2.0 mL of the test suspension to tubes T and Tb.
Add 5.0 mL of a 50 g/L solution of trichloroacetic acid R to tubes B, S1b, S2b, S3b and Tb. Mix by shaking.
Place the tubes and the casein solution in a water-bath at 35 ± 0.5 °C. Place a glass rod in each tube. When temperature equilibrium is reached, add 2.0 mL of the casein solution to tubes B, S1b, S2b, S3b and Tb. Mix. At time zero, add 2.0 mL of casein solution successively and at intervals of 30 s to tubes S1, S2, S3 and T. Mix immediately after each addition. Exactly 30 min after addition of the casein solution, taking into account the regular interval adopted, add 5.0 mL of a 50 g/L solution of trichloroacetic acid R to tubes S1, S2, S3 and T. Mix. Withdraw the tubes from the water-bath and allow to stand at room temperature for 20 min.
Filter the contents of each tube twice through the same suitable filter paper previously washed with a 50 g/L solution of trichloroacetic acid R, then with water R and dried.
A suitable filter paper complies with the following test: filter 5 mL of a 50 g/L solution of trichloroacetic acid R on a 7 cm disc of white filter paper; the absorbance (2.2.25) of the filtrate, measured at 275 nm using unfiltered trichloroacetic acid solution as the compensation liquid, is less than 0.04.
A schematic presentation of the above operations is shown in Table 0350.-1.
Table 0350.-1
| Tubes | |||||||||
| S1 | S1b | S2 | S2b | S3 | S3b | T | Tb | B | |
| Buffer solution | 2 | 2 | 1 | 1 | 1 | 1 | 3 | ||
| Reference suspension | 1 | 1 | 2 | 2 | 3 | 3 | |||
| Test suspension | 2 | 2 | |||||||
| Trichloroacetic acid solution | 5 | 5 | 5 | 5 | 5 | ||||
| Mix | + | + | + | + | + | ||||
| Water-bath 35 °C | + | + | + | + | + | + | + | + | + |
| Casein solution | 2 | 2 | 2 | 2 | 2 | ||||
| Mix | + | + | + | + | + | ||||
| Casein solution | 2 | 2 | 2 | 2 | |||||
| Mix | + | + | + | + | |||||
| Water-bath 35 °C 30 min | + | + | + | + | + | + | + | + | |
| Trichloroacetic acid solution | 5 | 5 | 5 | 5 | |||||
| Mix | + | + | + | + | |||||
| Room temperature 20 min |
+ | + | + | + | + | + | + | + | + |
| Filter | + | + | + | + | + | + | + | + | + |
Measure the absorbance (2.2.25) of the filtrates at 275 nm using the filtrate obtained from tube B as the compensation liquid.
Correct the average absorbance values for the filtrates obtained from tubes S1, S2 and S3 by subtracting the average values obtained for the filtrates from tubes S1b, S2b and S3b respectively. Draw a calibration curve of the corrected values against the volume of reference suspension used.
Determine the activity of the substance to be examined using the corrected absorbance for the test suspension (T – Tb) and the calibration curve and taking into account the dilution factors.
The test is not valid unless the corrected absorbance values are between 0.15 and 0.60.
Lipolytic activity
The lipolytic activity is determined by comparing the rate at which a suspension of pancreas powder hydrolyses a substrate of olive oil emulsion with the rate at which a suspension of pancreas powder (lipase) BRP hydrolyses the same substrate under the same conditions. The test is carried out under nitrogen.
Olive oil stock emulsion In an 800 mL beaker 9 cm in diameter, place 40 mL of olive oil R, 330 mL of acacia solution R and 30 mL of water R. Place an electric mixer at the bottom of the beaker. Place the beaker in a vessel containing ethanol (96 per cent) R and a sufficient quantity of ice as a cooling mixture. Emulsify using the mixer at an average speed of 1000-2000 r/min. Cool to 5-10 °C. Increase the mixing speed to 8000 r/min. Mix for 30 min keeping the temperature below 25 °C by the continuous addition of crushed ice into the cooling mixture. (A mixture of calcium chloride and crushed ice is also suitable). Store the stock emulsion in a refrigerator and use within 14 days. The emulsion must not separate into 2 distinct layers. Check the diameter of the globules of the emulsion under a microscope. At least 90 per cent have a diameter below 3 μm and none has a diameter greater than 10 μm. Shake the emulsion thoroughly before preparing the emulsion substrate.
Olive oil emulsion For 10 determinations, mix the following solutions in the order indicated: 100 mL of the stock emulsion, 80 mL of tris(hydroxymethyl)aminomethane solution R1, 20 mL of a freshly prepared 80 g/L of sodium taurocholate BRP and 95 mL of water R. Use on the day of preparation.
Apparatus Use a reaction vessel of about 50 mL capacity provided with:
— a device that will maintain a temperature of 37 ± 0.5 °C;
— a magnetic stirrer;
— a lid with holes for the insertion of electrodes, the tip of a burette, a tube for the admission of nitrogen and the introduction of reagents.
An automatic or manual titration apparatus may be used. In the latter case, the burette is graduated in 0.005 mL and the pH-meter is provided with a wide reading scale and glass-silver-silver chloride or other suitable electrodes. After each test the reaction vessel is evacuated by suction and washed several times with water R, the washings being removed each time by suction.
To avoid absorption of water formed by condensation, allow the preparation to be examined and the reference preparation to reach room temperature before opening the containers.
For the test suspension and the reference suspension, prepare the suspension and carry out the dilution at 0-4 °C.
Test suspension: In a small mortar cooled to 0-4 °C, triturate carefully a quantity of the substance to be examined equivalent to about 2500 Ph. Eur. U. of lipolytic activity with 1 mL of maleate buffer solution pH 7.0 R (lipase solvent) until a very fine suspension is obtained. Dilute the suspension with maleate buffer solution pH 7.0 R, transfer quantitatively to a volumetric flask and dilute to 100.0 mL with the buffer solution. Keep the flask containing the test suspension in iced water during the titration.
Reference suspension: Prepare a suspension of pancreas powder (lipase) BRP as described for the test suspension using a quantity equivalent to about 2500 Ph. Eur. U.
Carry out the titrations immediately after preparation of the test suspension and the reference suspension. Place 29.5 mL of olive oil emulsion in the reaction vessel equilibrated at 37 ± 0.5 °C. Fit the vessel with the electrodes, a stirrer and the burette (the tip being immersed in the olive oil emulsion).
Put the lid in place and switch on the apparatus. Carefully add 0.1 M sodium hydroxide with stirring to adjust to pH 9.2. Using a rapid-flow graduated pipette transfer about 0.5 mL of the previously homogenised reference suspension, start the chronometer and add continuously 0.1 M sodium hydroxide to maintain the pH at 9.0. After exactly 1 min, note the volume of 0.1 M sodium hydroxide used. Carry out the measurement a further 4 times. Discard the first reading and determine the
average of the 4 others (S1). Make 2 further determinations (S2 and S3). Calculate the average of the values S1, S2 and S3.
The average volume of 0.1 M sodium hydroxide used should be about 0.12 mL per minute with limits of 0.08 mL to 0.16 mL.
Carry out 3 determinations in the same manner for the test suspension (T1, T2 and T3). If the quantity of 0.1 M sodium hydroxide used is outside the limits of 0.08 mL to 0.16 mL per minute, the assay is repeated with a quantity of test suspension that is more suitable but situated between 0.4 mL and 0.6 mL. Otherwise the quantity of the substance to be examined is adjusted to comply with the conditions of the test. Calculate the average of the values T1, T2 and T3.
Calculate the activity in European Pharmacopoeia Units per milligram using the following expression:
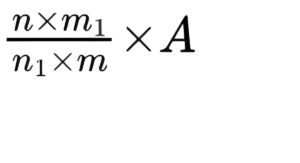
n = average volume of 0.1 M sodium hydroxide used per minute during the titration of the test suspension, in millilitres;
n1 = average volume of 0.1 M sodium hydroxide used per minute during the titration of the reference suspension, in millilitres;
m = mass of the substance to be examined, in milligrams;
m1 = mass of the reference preparation, in milligrams;
A = activity of pancreas powder (lipase) BRP, in European Pharmacopoeia Units per milligram.
Amylolytic activity
The amylolytic activity is determined by comparing the rate at which a suspension of pancreas powder hydrolyses a substrate of starch solution with the rate at which a suspension of pancreas powder (amylase) BRP hydrolyses the same substrate under the same conditions.
Starch solution To a quantity of starch BRP equivalent to 2.0 g of the dried substance add 10 mL of water R and mix. (Determine the water content of starch BRP prior to the test by heating at 120 °C for 4 h). Add this suspension, whilst stirring continuously, to 160 mL of boiling water R. Wash the container several times with successive quantities, each of 10 mL, of water R and add the washings to the hot starch solution. Heat to boiling, stirring continuously. Cool to room temperature and dilute to 200 mL with water R. Use the solution on the day of preparation.
To avoid absorption of water formed by condensation, allow the preparation to be examined and the reference preparation to reach room temperature before opening the containers.
For the test suspension and the reference suspension, prepare the suspension and carry out the dilution at 0-4 °C.
Test suspension: Triturate a quantity of the substance to be examined equivalent to about 1500 Ph. Eur. U. of amylolytic activity with 60 mL of phosphate buffer solution pH 6.8 R1 for 15 min. Transfer quantitatively to a volumetric flask and dilute to 100.0 mL with phosphate buffer solution pH 6.8 R1.
Reference suspension: Prepare a suspension of pancreas powder (amylase) BRP as described for the test suspension, using a quantity equivalent to about 1500 Ph. Eur. U.
In a test-tube 200 mm long and 22 mm in diameter, fitted with a ground-glass stopper, place 25.0 mL of starch solution, 10.0 mL of phosphate buffer solution pH 6.8 R1 and 1.0 mL of an 11.7 g/L solution of sodium chloride R. Close the tube, shake and place in a water-bath at 25.0 ± 0.1 °C. When the temperature equilibrium has been reached, add 1.0 mL of the test suspension and start the chronometer. Mix and place the tube in the water-bath. After exactly 10 min, add 2 mL of 1 M hydrochloric acid. Transfer the mixture quantitatively to a 300 mL conical flask fitted with a ground-glass stopper. Whilst shaking continuously, add 10.0 mL of 0.05 M iodine immediately followed by 45 mL of 0.1 M sodium hydroxide. Allow to stand in the dark at a temperature between 15 °C and 25 °C for 15 min. Add 4 mL of a mixture of 1 volume of sulfuric acid R and 4 volumes of water R. Titrate the excess of iodine with 0.1 M sodium thiosulfate using a microburette. Carry out a blank titration adding the 2 mL of 1 M hydrochloric acid before introducing the test suspension. Carry out the titration of the reference suspension in the same manner.
The test is not valid unless both n′-n and n′1-n1 are between 1.9 mL and 3.6 mL.
Calculate the amylolytic activity in European Pharmacopoeia Units per milligram using the following expression:
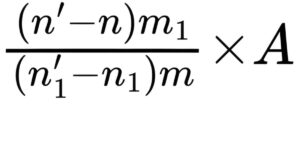
n = volume of 0.1 M sodium thiosulfate used in the titration of the test suspension, in millilitres;
n1 = volume of 0.1 M sodium thiosulfate used in the titration of the reference suspension, in millilitres;
n′ = volume of 0.1 M sodium thiosulfate used in the blank titration of the test suspension, in millilitres;
n′1 = volume of 0.1 M sodium thiosulfate used in the blank titration of the reference suspension, in millilitres;
m = mass of the substance to be examined, in milligrams;
m1 = mass of the reference preparation, in milligrams;
A = activity of pancreas powder (amylase) BRP, in European Pharmacopoeia Units per milligram.
STORAGE
In an airtight container.






