(Ph. Eur. monograph 3098)
O2 32.00
Action and use
Oxygen supplementation for medical emergencies.
DEFINITION
Oxygen produced in a two-stage adsorption plant, applying differential pressures to the adsorption vessels, using different zeolites / molecular sieves to reduce the levels of nitrogen and argon in the ambient air.
Content
Minimum 96.0 per cent V/V of O2, the remainder consists mainly of argon and nitrogen.
This monograph does not apply to gas produced using individual concentrators for domiciliary use.
CHARACTERS
Appearance
Colourless gas.
IDENTIFICATION
It complies with the limits of the assay.
TESTS
Carbon dioxide
Maximum 300 ppm V/V, continuously determined using an infrared analyser (2.5.24).
Gas to be examined: The substance to be examined. It must be filtered to avoid stray light phenomena.
Reference gas (a): A mixture of 2 per cent V/V of nitrogen R1 and 98 per cent V/V of oxygen R.
Reference gas (b): A mixture of 2 per cent V/V of nitrogen R1 and 98 per cent V/V of oxygen R, containing 300 ppm V/V of carbon dioxide R1.
Calibrate the apparatus and set the sensitivity using reference gases (a) and (b). Measure the content of carbon dioxide in the gas to be examined.
Carbon monoxide
Maximum 5 ppm V/V, continuously determined using an infrared analyser (2.5.25).
Gas to be examined: The substance to be examined. It must be filtered to avoid stray light phenomena.
Reference gas (a): A mixture of 2 per cent V/V of nitrogen R1 and 98 per cent V/V of oxygen R.
Reference gas (b): A mixture containing 5 ppm V/V of carbon monoxide R in nitrogen R1.
Calibrate the apparatus and set the sensitivity using reference gases (a) and (b). Measure the content of carbon monoxide in the gas to be examined.
Nitrogen monoxide and nitrogen dioxide
Maximum 2 ppm V/V in total, determined using a chemiluminescence analyser (2.5.26).
Gas to be examined: The substance to be examined.
Reference gas (a): A mixture of 2 per cent V/V of nitrogen R1 and 98 per cent V/V of oxygen R, containing less than 0.05 ppm V/V of nitrogen monoxide and nitrogen dioxide.
Reference gas (b): A mixture of 2 per cent V/V of nitrogen R1 and 98 per cent V/V of oxygen R, containing 2 ppm V/V of nitrogen dioxide R.
Calibrate the apparatus and set the sensitivity using reference gases (a) and (b). Measure the content of nitrogen monoxide and nitrogen dioxide in the gas to be examined.
Sulfur dioxide
Maximum 1 ppm V/V, determined using an ultraviolet fluorescence analyser (Figure 3098.-1).
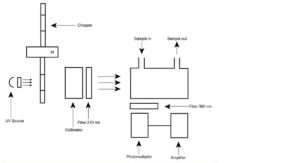
Figure 3098.-1. – UV fluorescence analyser
The apparatus consists of the following:
— a system generating ultraviolet radiation with a wavelength of 210 nm, made up of an ultraviolet lamp, a collimator, and a selective filter; the beam is blocked periodically by a chopper rotating at high speeds;
— a reaction chamber, through which flows the gas to be examined;
— a system that detects radiation emitted at a wavelength of 350 nm, made up of a selective filter, a photomultiplier tube and an amplifier.
Gas to be examined: The substance to be examined. It must be filtered.
Reference gas (a): A mixture of 2 per cent V/V of nitrogen R1 and 98 per cent V/V of oxygen R.
Reference gas (b): A mixture of 2 per cent V/V of nitrogen R1 and 98 per cent V/V of oxygen R, containing 0.5 ppm V/V to 2 ppm V/V of sulfur dioxide R1.
Calibrate the apparatus and set the sensitivity using reference gases (a) and (b). Measure the content of sulfur dioxide in the gas to be examined.
Oil
Maximum 0.1 mg/m3 , determined using an oil detector tube (2.1.6).
Water
Maximum 67 ppm V/V, continuously determined using an electrolytic hygrometer (2.5.28).
Other impurities
A risk assessment must be performed to identify any additional impurities that may be present in the gas produced by the two-stage adsorption plant.
Where applicable, special consideration should be given to identifying any potential impurities that could cause harm to the patient, related to:
— the environment in which the plant is situated;
— any emissions within the plant that could lead to impurities;
— compression of the oxygen when filling high-pressure cylinders.
When indicated by the risk assessment, some or all of these impurities may need to be continuously monitored.
ASSAY
Continuously determine the content of oxygen using a paramagnetic analyser (2.5.27).
STORAGE
Storage requirements are not applicable where Oxygen (98 per cent) is produced by an on-site adsorption plant that feeds the gas directly into a medical gas pipeline system (via buffer vessels) for delivery to the patient.
Where the surplus gas produced is compressed into a high-pressure vessel / manifolded cylinders, for use as a reserve supply to cover variability in demand or as a backup supply, it may be stored in suitable containers complying with relevant national pressure equipment regulations. Oils and grease are not to be used unless they are oxygen-compatible.
LABELLING
When the gas produced is stored, the label states the nominal content of O2 in per cent V/V.
IMPURITIES
A. CO2: carbon dioxide;
B. CO: carbon monoxide;
C. SO2: sulfur dioxide;
D. NO and NO2: nitrogen monoxide and nitrogen dioxide;
E. oil;
F. H2O: water.






