Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Antihelminthic.
Preparation
Oxyclozanide Oral Suspension
DEFINITION
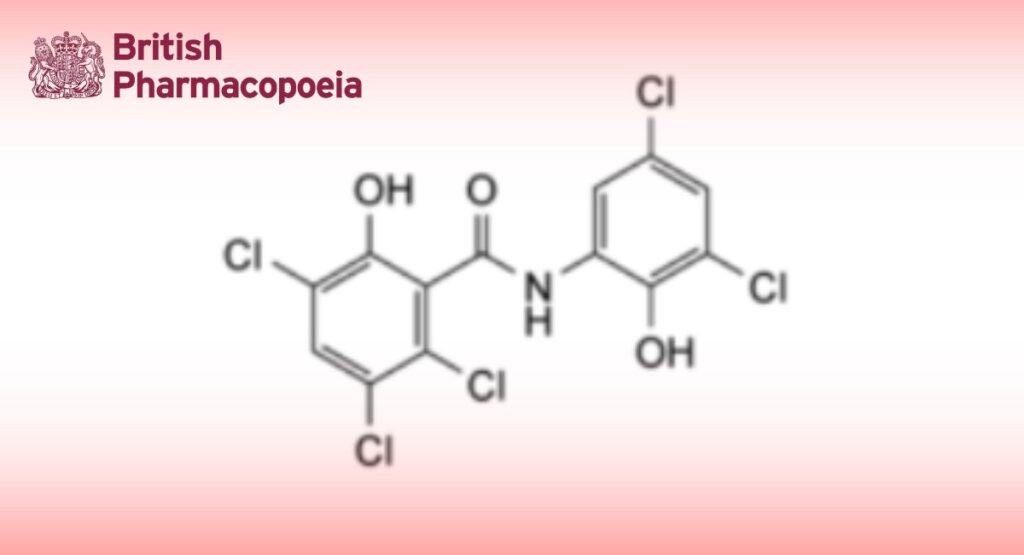
Oxyclozanide is 3,3′,5,5′,6-pentachloro-2′-hydroxysalicylanilide. It contains not less than 98.0% and not more than 101.0% of C13H6Cl5NO3, calculated with reference to the dried substance.
CHARACTERISTICS
A pale cream or cream coloured powder.
Very slightly soluble in water; freely soluble in acetone; soluble in ethanol (96%); slightly soluble in chloroform.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of oxyclozanide (RSV 33).
B. The light absorption, Appendix II B, in the range 250 to 350 nm of a 0.003% w/v solution in acidified methanol exhibits a maximum only at 300 nm. The absorbance at the maximum is about 0.76, Appendix II B.
C. Melting point, about 208°, Appendix V A.
TESTS
Ionisable chlorine
Dissolve 2 g in 100 mL of methanol, add 10 mL of 1.5M nitric acid and titrate with 0.1M silver nitrate VS determining the end point potentiometrically. Not more than 1.4 mL is required (0.25%).
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) 0.1% w/v of the substance being examined prepared by dissolving it in a suitable volume of methanol and slowly diluting with water containing 0.1% v/v orthophosphoric acid to give a solution containing about the same ratio of methanol to water as the mobile phase.
(2) Dilute 1 volume of solution (1) to 100 volumes with the mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (20 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Hypersil ODS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 300 nm.
(f) Inject 20 µL of each solution.
MOBILE PHASE
A mixture of methanol and water containing 0.1% v/v of orthophosphoric acid (a mixture of 38 volumes of water and 62 volumes of methanol is usually suitable).
LIMITS
In the chromatogram obtained with solution (1):
the area of any secondary peak with a retention time less than that of the principal peak is not greater than one third of the area of the principal peak in the chromatogram obtained with solution (2) (0.3%);
the area of any secondary peak with a retention time greater than that of the principal peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1%).
Loss on drying
When dried to constant weight at 60° at a pressure not exceeding 0.7 kPa, loses not more than 1.0% of its weight. Use 1 g.
Sulfated ash
Not more than 0.2%, Appendix IX A.
ASSAY
Dissolve 0.25 g in 75 mL of anhydrous pyridine and pass a current of nitrogen through the solution for 5 minutes. Carry out Method II for non-aqueous titration, Appendix VIII A, maintaining a current of nitrogen through the solution throughout the titration, using 0.1M tetrabutylammonium hydroxide VS as titrant and determining the end point potentiometrically. Each mL of 0.1M tetrabutylammonium hydroxide VS is equivalent to 20.07 mg of C13H6Cl5NO3.