Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Local anaesthetic.
DEFINITION
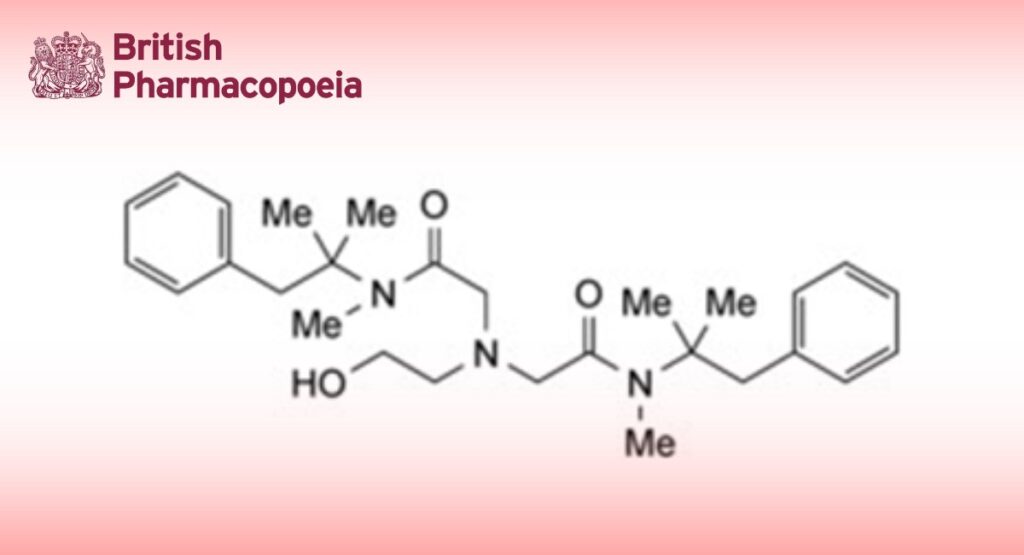
Oxetacaine is 2,2′-(2-hydroxyethylimino)bis[N-(α,α-dimethylphenethyl)-N-methylacetamide]. It contains not less than 99.0% and not more than 100.5% of C28H41N3O3, calculated with reference to the dried substance.
CHARACTERISTICS
A white or almost white powder.
Practically insoluble in water; freely soluble in methanol; soluble in ethyl acetate.
IDENTIFICATION
The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of oxetacaine (RS 254).
TESTS
Melting point
100°C to 104°C, Appendix V A.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in ethyl acetate.
(1) 10.0% w/v of the substance being examined.
(2) Dilute 1 volume of solution (1) to 200 volumes.
(3) Dilute 1 volume of solution (2) to 5 volumes.
CHROMATOGRAPHIC CONDITIONS
(a) Use a silica gel 60 precoated plate (Merck plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 5 µL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry it in a current of warm air and spray liberally with a solution containing 6% w/v of ammonium thiocyanate and 2% w/v of cobalt(II) chloride. Carefully remove excess solution by applying filter paper to the plate and allow the plate to dry in air for 10 minutes or until spots appear.
MOBILE PHASE
1 volume of 18M ammonia, 20 volumes of absolute ethanol and 79 volumes of toluene.
LIMITS
In the chromatogram obtained with solution (1):
any secondary spot is not more intense than the spot in the chromatogram obtained with solution (2) (0.5%);
not more than one secondary spot is more intense than the spot in the chromatogram obtained with solution (3) (0.1%).
Loss on drying
When dried at 60°C at a pressure not exceeding 0.7 kPa for 4 hours, loses not more than 0.5% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Dissolve 1 g in 50 mL of anhydrous acetic acid and carry out Method I for non-aqueous titration, Appendix VIII A, determining the end-point potentiometrically. Each mL of 0.1M perchloric acid VS is equivalent to 46.76 mg of C28H41N3O3.