Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Penicillin antibacterial.
Ph Eur
DEFINITION
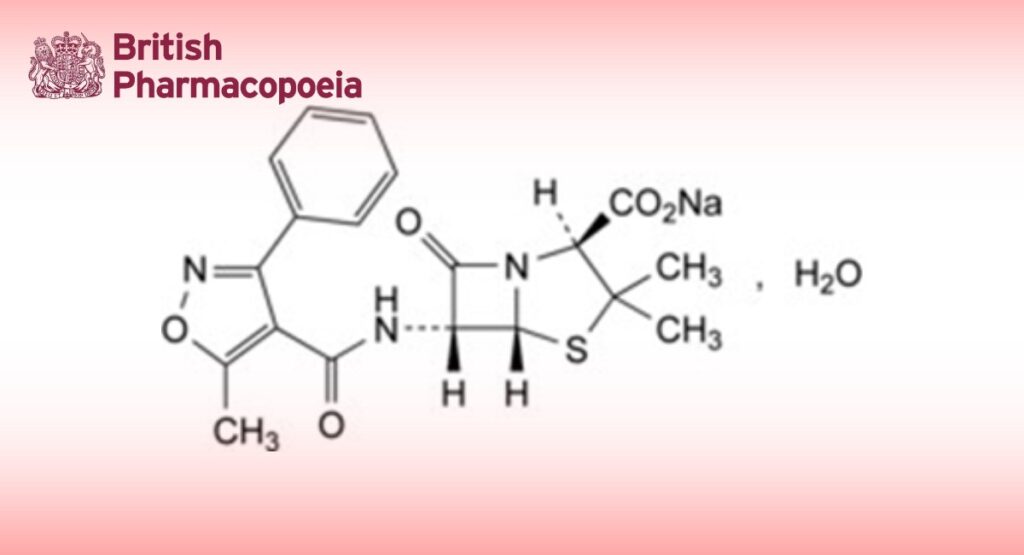
Sodium (2S,5R,6R)-3,3-dimethyl-6-[[(5-methyl-3-phenylisoxazol-4-yl)carbonyl]amino]-7-oxo-4-thia-1- azabicyclo[3.2.0]heptane-2-carboxylate monohydrate.
Semi-synthetic product derived from a fermentation product.
Content
95.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Freely soluble in water, soluble in methanol, practically insoluble in methylene chloride.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison oxacillin sodium monohydrate CRS.
B. It gives reaction (a) of sodium (2.3.1).
TESTS
Appearance of solution
The solution is clear (2.2.1) and its absorbance (2.2.25) at 430 nm is not greater than 0.10. Dissolve 2.50 g in water R and dilute to 25.0 mL with the same solvent.
pH (2.2.3)
4.5 to 7.5.
Dissolve 0.30 g in carbon dioxide-free water R and dilute to 10 mL with the same solvent.
Specific optical rotation (2.2.7)
+ 196 to + 212 (anhydrous substance).
Dissolve 0.250 g in water R and dilute to 25.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution (a) Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 50.0 mL with the mobile phase.
Test solution (b) Dilute 5.0 mL of test solution (a) to 50.0 mL with the mobile phase.
Reference solution (a) Dissolve 50.0 mg of oxacillin sodium monohydrate CRS in the mobile phase and dilute to 50.0 mL with the mobile phase. Dilute 5.0 mL of the solution to 50.0 mL with the mobile phase.
Reference solution (b) Dilute 5.0 mL of test solution (b) to 50.0 mL with the mobile phase.
Reference solution (c) Dissolve 5 mg of cloxacillin sodium CRS (impurity E) and 5 mg of oxacillin sodium monohydrate CRS in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (d) In order to prepare impurities B and D in situ, dissolve 25 mg of the substance to be examined in 1 mL of 0.05 M sodium hydroxide, allow to stand for 3 min, then dilute to 100 mL with the mobile phase. Inject immediately.
Reference solution (e) Dissolve 5 mg of oxacillin for peak identification CRS (containing impurities E, F, G, I and J) in 5 mL of the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.0 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase Mix 25 volumes of acetonitrile R and 75 volumes of a 2.7 g/L solution of potassium dihydrogen phosphate R previously adjusted to pH 5.0 with dilute sodium hydroxide solution R.
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 225 nm.
Injection 20 µL of test solution (a) and reference solutions (b), (c), (d) and (e).
Run time 7 times the retention time of oxacillin.
Identification of impurities:
— in the chromatogram obtained with reference solution (d), the 2 principal peaks eluting before the main peak are due to impurities B and D respectively;
— use the chromatogram supplied with oxacillin for peak identification CRS and the chromatogram obtained with reference solution (e) to identify the peaks due to impurities E, F, G, I and J.
Relative retention With reference to oxacillin (retention time = about 5 min): impurity A = about 0.3; impurity B (isomer 1) = about 0.4; impurity B (isomer 2) = about 0.5; impurity C = about 0.65; impurity D (2 epimers) = about 0.9; impurity E = about 1.5; impurity F = about 1.9; impurity G = about 2.1; impurity I = about 3.8; impurity J = about 5.8.
System suitability:
— resolution: minimum 2.5 between the peaks due to oxacillin and impurity E in the chromatogram obtained with reference solution (c);
— the chromatogram obtained with reference solution (e) is similar to the chromatogram supplied with oxacillin for peak identification CRS. Limits:
— impurity B: for the sum of the areas of the 2 isomer peaks, not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (1.5 per cent);
— impurity E: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (1.0 per cent);
— impurities D (sum of the 2 epimers), F, G, I, J: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— any other impurity: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— total: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (3.0 per cent);
— disregard limit: 0.05 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Ethyl acetate and butyl acetate
Head-space gas chromatography (2.2.28).
Test solution Dissolve 0.200 g of the substance to be examined in 6.0 mL of water R.
Reference solution Dissolve 83 mg of butyl acetate R and 83 mg of ethyl acetate R in water R and dilute to 250.0 mL with the same solvent. Use 6.0 mL of this solution.
Close the vials immediately with a rubber membrane stopper coated with polytetrafluoroethylene and secured with an aluminium crimp cap. Mix to obtain a homogeneous solution.
Column:
— material: fused silica;
— size: l = 50 m, Ø = 0.32 mm;
— stationary phase: methylpolysiloxane R (film thickness 5 µm).
Carrier gas helium for chromatography R. Flow rate 2 mL/min.
Static head-space conditions that may be used:
— equilibration temperature: 80 °C;
— equilibration time: 60 min;
— transfer-line temperature: 140 °C;
— pressurisation time: 30 s.
Temperature:
| Time (min) | Temperature (°C) | |
| Column | 0 – 6 | 70 |
| 6 – 16 | 70 → 220 | |
| 16 – 18 | 220 | |
| Injection port | 140 | |
| Detector | 250 | |
Detection Flame ionisation.
Retention time Ethyl acetate = about 10 min; butyl acetate = about 15.5 min.
Limits:
— butyl acetate: maximum 1.0 per cent;
— ethyl acetate: maximum 1.0 per cent.
N,N-Dimethylaniline (2.4.26, Method B) Maximum 20 ppm.
2- Ethylhexanoic acid (2.4.28) Maximum 0.8 per cent.
Water (2.5.12)
3- 5 per cent to 5.0 per cent, determined on 0.300 g.
Bacterial endotoxins (2.6.14)
Less than 0.20 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection Test solution (b) and reference solution (a).
Calculate the percentage content of C19H18N3NaO5S taking into account the assigned content of oxacillin sodium monohydrate CRS.
IMPURITIES
Specified impurities B, D, E, F, G, I, J.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.
Control of impurities in substances for pharmaceutical use) A, C.
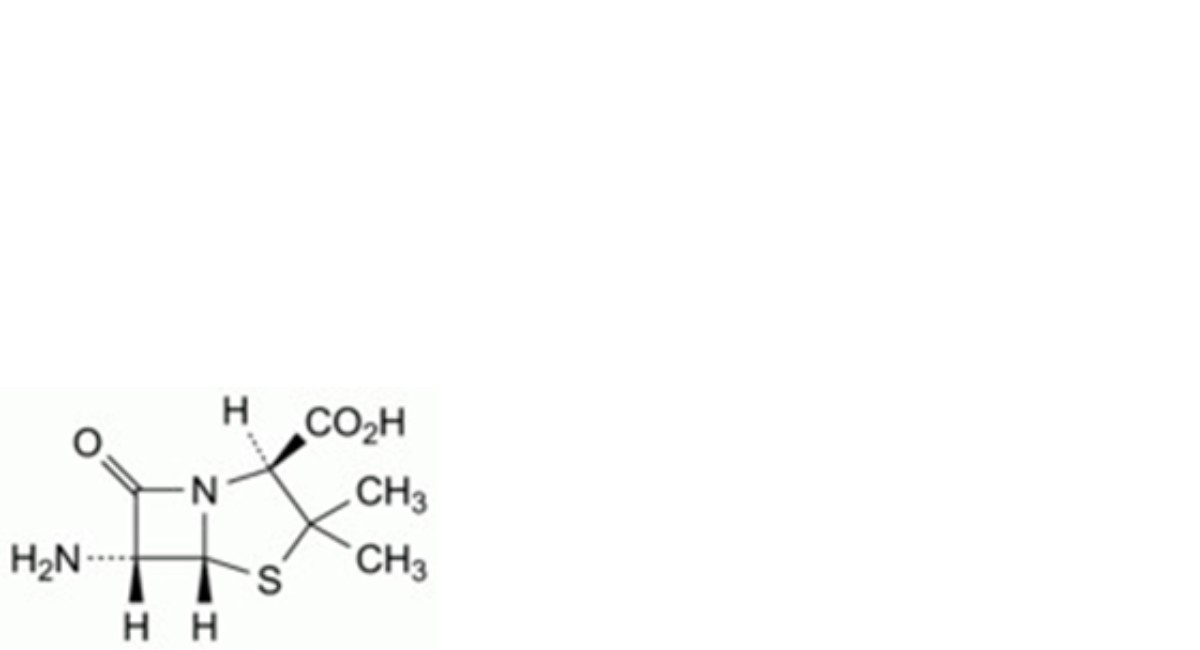
A. (2S,5R,6R)-6-amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (6- aminopenicillanic acid),
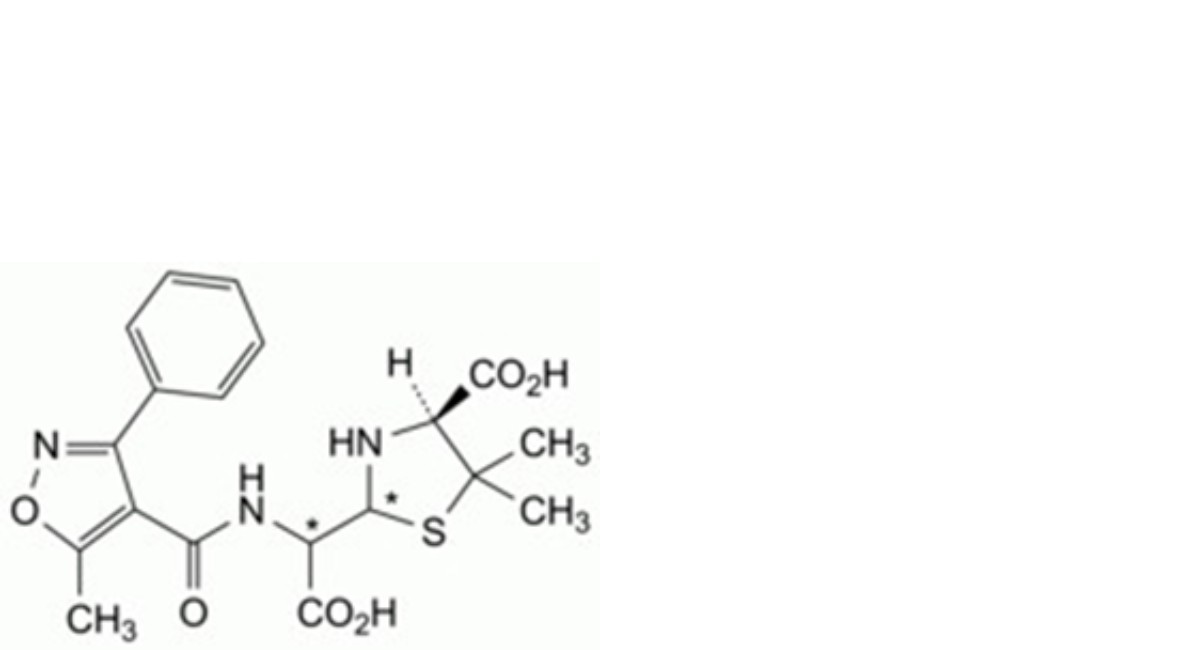
B. (4S)-2-[carboxy[[(5-methyl-3-phenylisoxazol-4-yl)carbonyl]amino]methyl]-5,5-dimethylthiazolidine-4- carboxylic acid (penicilloic acids of oxacillin),

C. 5-methyl-3-phenylisoxazole-4-carboxylic acid,
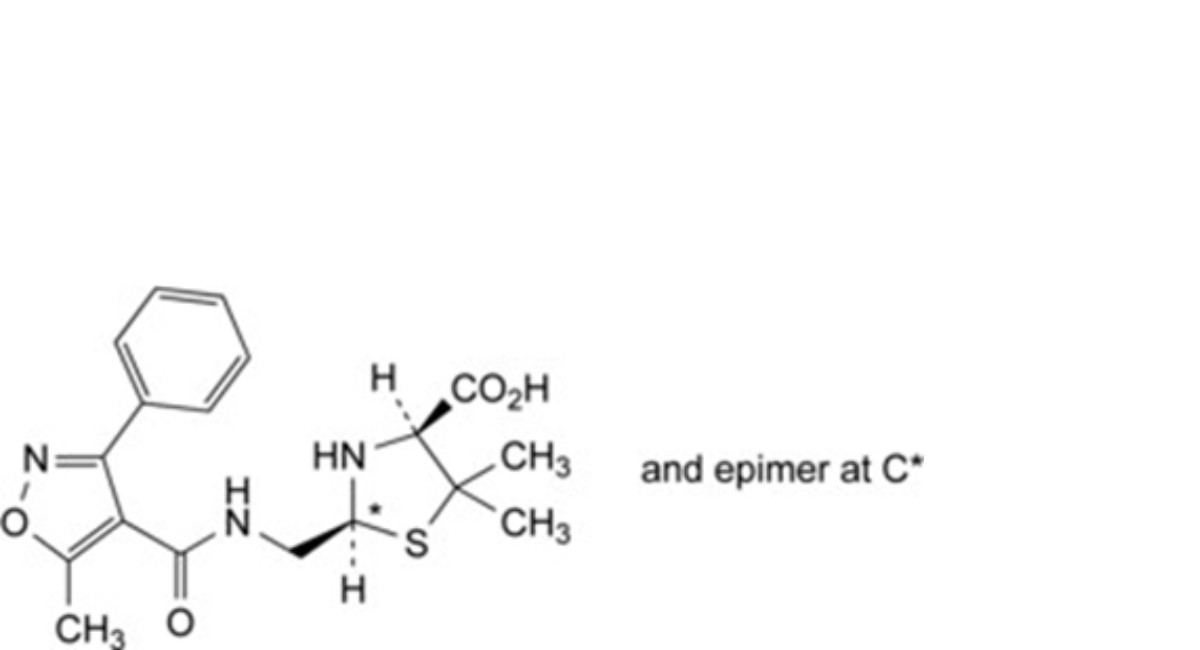
D. (2RS,4S)-5,5-dimethyl-2-[[[(5-methyl-3-phenylisoxazol-4-yl)carbonyl]amino]methyl]thiazolidine-4- carboxylic acid (penilloic acids of oxacillin),
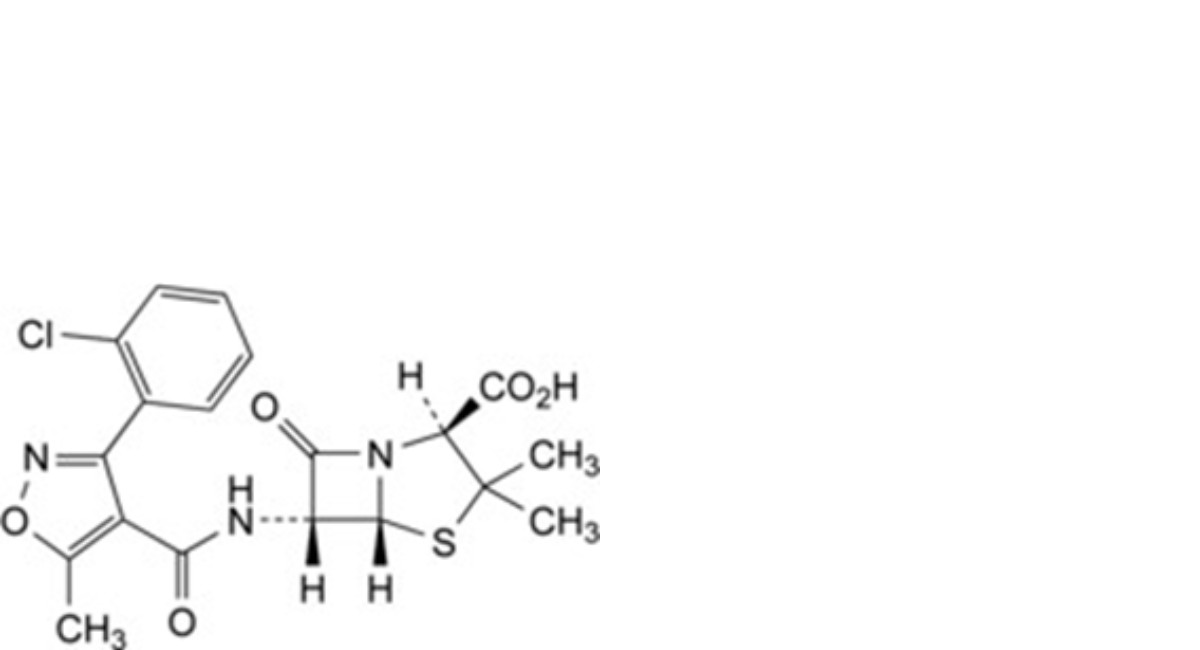
E. (2S,5R,6R)-6-[[[3-(2-chlorophenyl)-5-methylisoxazol-4-yl]carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1- azabicyclo[3.2.0]heptane-2-carboxylic acid (cloxacillin),
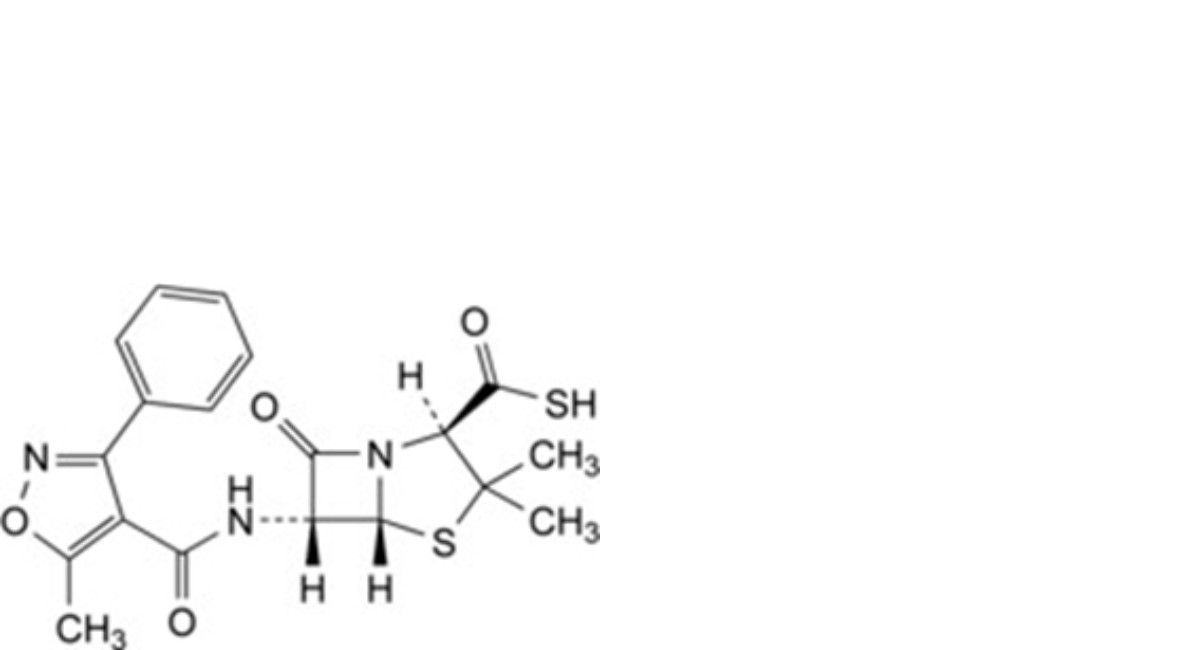
F. (2R,5R,6R)-3,3-dimethyl-6-[[(5-methyl-3-phenylisoxazol-4-yl)carbonyl]amino]-7-oxo-4-thia-1- azabicyclo[3.2.0]heptane-2-carbothioic acid (thiooxacillin),
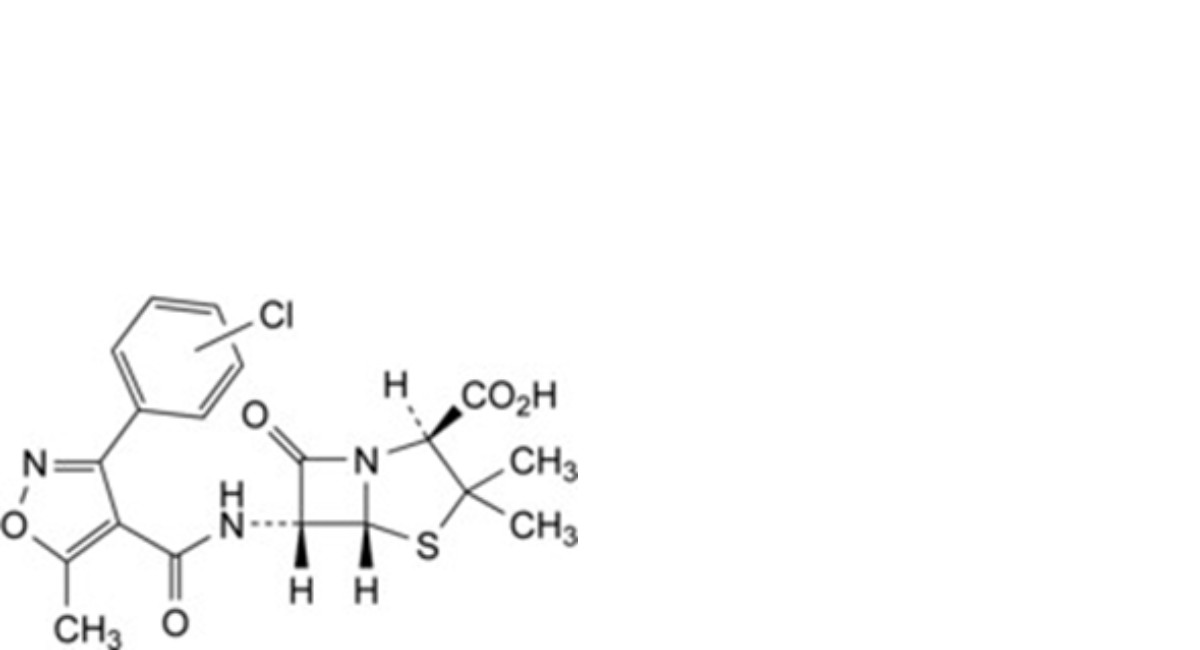
G. (2S,5R,6R)-6-[[[3-(chlorophenyl)-5-methylisoxazol-4-yl]carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1- azabicyclo[3.2.0]heptane-2-carboxylic acid (cloxacillin isomer),
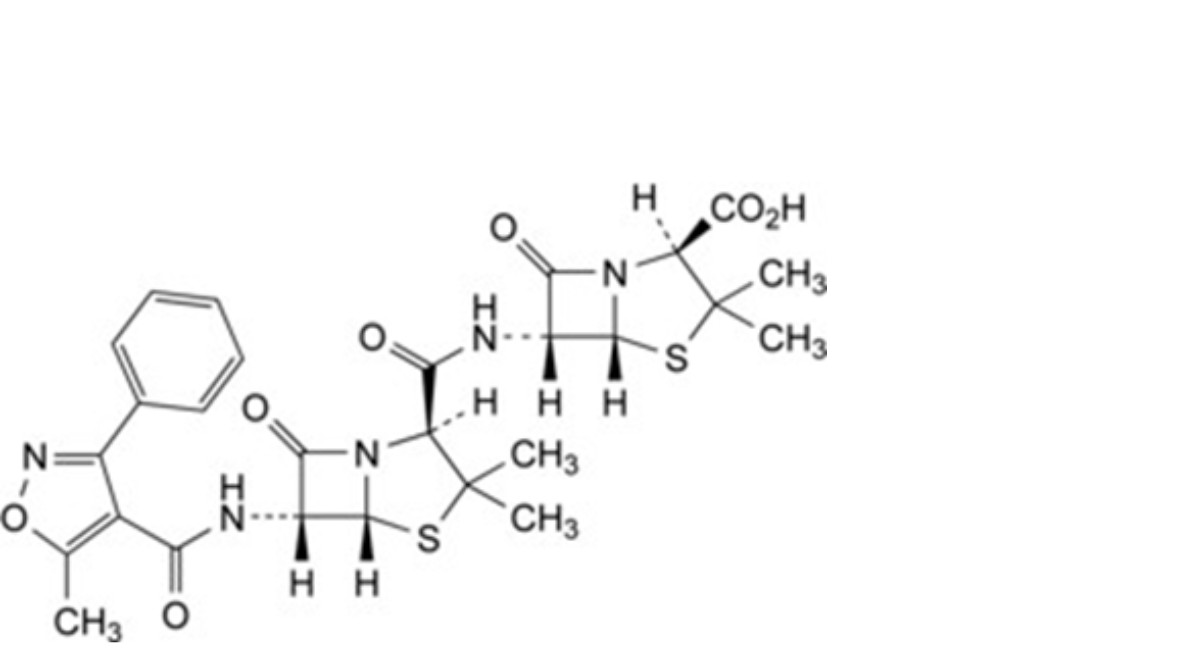
I. (2S,5R,6R)-6-[[(2S,5R,6R)-3,3-dimethyl-6-[[(5-methyl-3-phenylisoxazol-4-yl)carbonyl]amino]-7-oxo-4- thia-1-azabicyclo[3.2.0]heptane-2-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2- carboxylic acid (6-APA oxacillin amide),
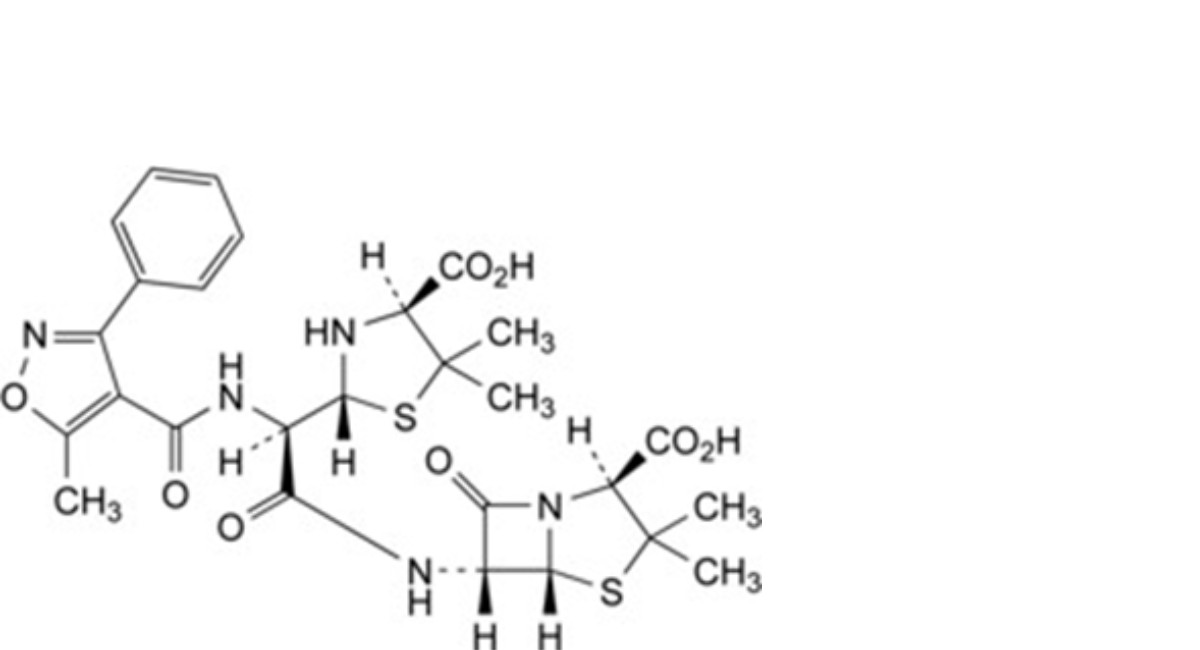
J. (2S,5R,6R)-6-[[(2R)-[(2R,4S)-4-carboxy-5,5-dimethylthiazolidin-2-yl][[(5-methyl-3-phenylisoxazol-4- yl)carbonyl]amino]acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (ozolamide of 6-APA dimer).
Ph Eur