Edition: BP 2025 (Ph. Eur. 11.6 update)
DEFINITION
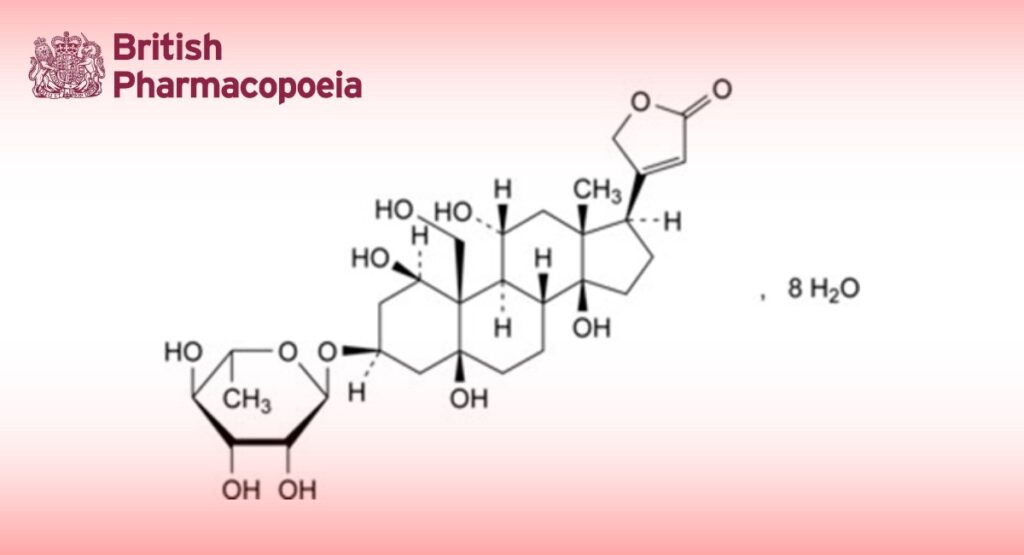
3β-[(6-Deoxy-α-L-mannopyranosyl)oxy]-1β,5,11α,14,19-pentahydroxy-5β,14β-card-20(22)-enolide octahydrate.
Content
96.0 per cent to 104.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or colourless crystals.
Solubility
Sparingly soluble in water and in anhydrous ethanol, practically insoluble in ethyl acetate.
IDENTIFICATION
A. Examine the chromatograms obtained in the test for related substances. The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the spot in the chromatogram obtained with reference solution (a).
B. Dissolve 2 mg to 3 mg in 2 mL of sulfuric acid R; a pink colour develops which quickly changes to red. The solution shows green fluorescence in ultraviolet light.
C. Dissolve about 1 mg in 1 mL of dinitrobenzene solution R and add 0.2 mL of dilute sodium hydroxide solution R. An intense blue colour develops.
D. Dissolve 0.1 g in 5 mL of a 150 g/L solution of sulfuric acid R and boil for a few minutes. The solution becomes yellow and turbid. Filter and add to the filtrate 5 mL of a 120 g/L solution of sodium hydroxide R and 3 mL of cupri-tartaric solution R. Heat. A red precipitate is formed.
TESTS
Solution S
Dissolve 0.20 g in 15 mL of water R, heating on a water-bath. Allow to cool and dilute to 20.0 mL with water R.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Specific optical rotation (2.2.7)
-33 to -30 (anhydrous substance), determined on solution S.
Related substances
Thin-layer chromatography (2.2.27).
Solvent mixture water R, chloroform R, methanol R (16:50:50 V/V/V).
Test solution Dissolve a quantity of the substance to be examined corresponding to 20 mg of the anhydrous substance in 1.0 mL of the solvent mixture.
Reference solution (a) Dissolve a quantity of ouabain CRS corresponding to 20 mg of the anhydrous substance in 1.0 mL of the solvent mixture.
Reference solution (b) Dissolve a quantity of ouabain CRS corresponding to 10 mg of the anhydrous substance in the solvent mixture and dilute to 25 mL with the solvent mixture.
Reference solution (c) Dilute 2.5 mL of reference solution (b) to 10 mL with the solvent mixture.
Plate TLC silica gel G plate R.
Mobile phase water R, dimethyl sulfoxide R, methanol R, chloroform R (4:15:15:70 V/V/V/V); homogenise the mixture before use.
Application 5 µL.
Development Over a path of 13 cm.
Drying Immediately at 140 °C for 30 min in a ventilated oven.
Detection Allow to cool, spray with alcoholic solution of sulfuric acid R and heat at 140 °C for 15 min.
System suitability:
— the principal spot in the chromatogram obtained with the test solution and the principal spot in the chromatogram obtained with reference solution (a) migrate over a distance sufficient to give unequivocal separation of the secondary spots;
— the chromatogram obtained with reference solution (c) shows a clearly visible spot.
Limit:
— any impurity: any spot in the chromatogram obtained with the test solution, apart from the principal spot, is not more intense than the spot in the chromatogram obtained with reference solution (b) (2.0 per cent).
Alkaloids and strophanthin-K
To 5.0 mL of solution S add 0.5 mL of a 100 g/L solution of tannic acid R. No precipitate is formed.
Water (2.5.12)
18.0 per cent to 22.0 per cent, determined on 0.100 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Test solution Dissolve 40.0 mg in ethanol (96 per cent) R and dilute to 50.0 mL with the same solvent. Dilute 5.0 mL of the solution to 100.0 mL with ethanol (96 per cent) R.
Reference solution Dissolve 40.0 mg of ouabain CRS in ethanol (96 per cent) R and dilute to 50.0 mL with the same solvent. Dilute 5.0 mL of the solution to 100.0 mL with ethanol (96 per cent) R.
To 5.0 mL of each solution add 3.0 mL of alkaline sodium picrate solution R, allow to stand protected from bright light for 30 min and measure the absorbance (2.2.25) of both solutions at the absorption maximum at 495 nm using as the compensation liquid a mixture of 3.0 mL of alkaline sodium picrate solution R and 5.0 mL of ethanol (96 per cent) R prepared at the same time.
Calculate the percentage content of C29H44O12 from the absorbances measured and the concentrations of the solutions.
STORAGE
Protected from light. Ph Eur