Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Anticholinergic.
Ph Eur
DEFINITION
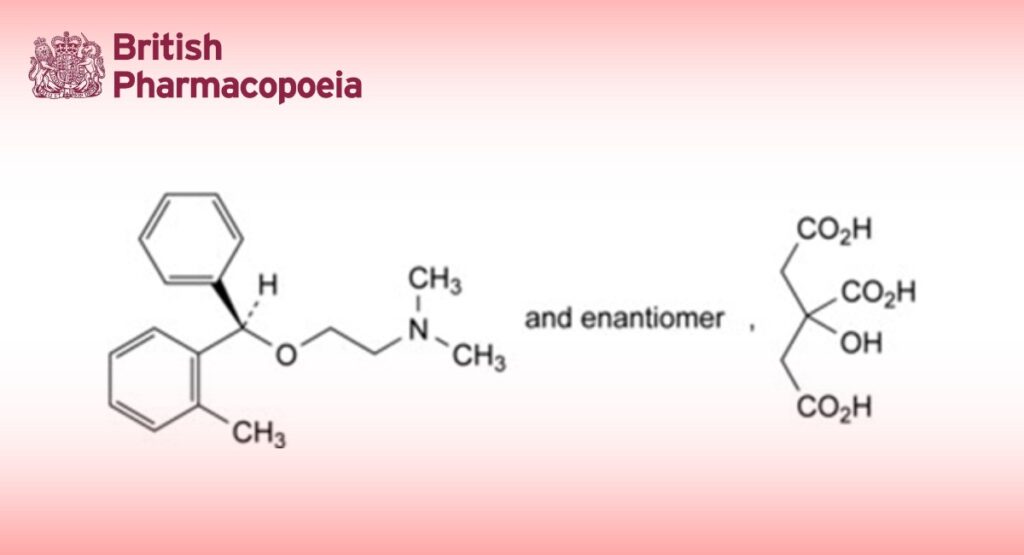
(RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3- tricarboxylate.
Content
98.5 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Sparingly soluble in water, slightly soluble in ethanol (96 per cent).
mp
About 137 °C.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison orphenadrine citrate CRS.
TESTS
Appearance of solution
The solution is clear (2.2.1) and its absorbance (2.2.25) at 436 nm has a maximum of 0.050.
Dissolve 1.0 g in a 3.6 per cent V/V solution of hydrochloric acid R in ethanol (96 per cent) R and dilute to 10.0 mL with the same acid solution.
Related substances
Gas chromatography (2.2.28): use the normalisation procedure.
Test solution Dissolve 0.500 g of the substance to be examined in water R and dilute to 50 mL with the same solvent. Add 2 mL of concentrated ammonia R and shake with 3 quantities, each of 10 mL, of toluene R. To the combined upper layers add anhydrous sodium sulfate R, shake, filter and evaporate the filtrate by suitable means, at a temperature not exceeding 50 °C. Take up the residue with toluene R and dilute to 20.0 mL with the same solvent.
Reference solution (a) Dissolve 30 mg of orphenadrine citrate CRS and 30 mg of orphenadrine impurity E CRS in 20 mL of water R. Add 1 mL of concentrated ammonia R and shake with 3 quantities, each of 5 mL, of toluene R. To the combined upper layers add anhydrous sodium sulfate R, shake, filter and evaporate the filtrate by suitable means, at a temperature not exceeding 50 °C. Take up the residue with toluene R and dilute to 20.0 mL with the same solvent.
Reference solution (b) Dissolve the contents of a vial of orphenadrine for peak identification CRS (containing impurities A, B, C, D and F) in 1.0 mL of toluene R.
Column:
— size: l = 60 m, Ø = 0.32 mm;
— stationary phase: phenyl(5)methyl(95)polysiloxane R (film thickness 1.0 µm).
Carrier gas helium for chromatography R. Flow rate 1 mL/min.
Split ratio 1:25.
Temperature:
— column: 240 °C;
— injection port and detector: 290 °C.
Detection Flame ionisation.
Injection 2 µL.
Run time 1.3 times the retention time of orphenadrine.
Identification of impurities Use the chromatogram supplied with orphenadrine for peak identification CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A, B, C, D and F. Use the chromatogram obtained with reference solution (a) to identify the peak due to impurity E.
Relative retention With reference to orphenadrine (retention time = about 13 min): impurity B = about 0.5; impurity A = about 0.6; impurity D = about 0.8; impurity C = about 0.9; impurity E = about 0.98; impurity F = about 1.1.
System suitability Reference solution (a):
— resolution: minimum of 1.5 between the peaks due to impurity E and orphenadrine.
Limits:
— impurities A, B, C, D, E, F: for each impurity, not more than 0.3 per cent;
— unspecified impurities: for each impurity, not more than 0.10 per cent;
— total: maximum 1.0 per cent;
— disregard limit: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 3 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.350 g in 50 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 46.15 mg of C24H31NO8.
STORAGE
Protected from light. If the substance is sterile, store in a sterile, airtight, tamper-evident container, protected from light.
IMPURITIES
Specified impurities A, B, C, D, E, F.

A. (RS)-(2-methylphenyl)phenylmethanol (2-methylbenzhydrol),

B. (2-methylphenyl)phenylmethanone (2-methylbenzophenone),
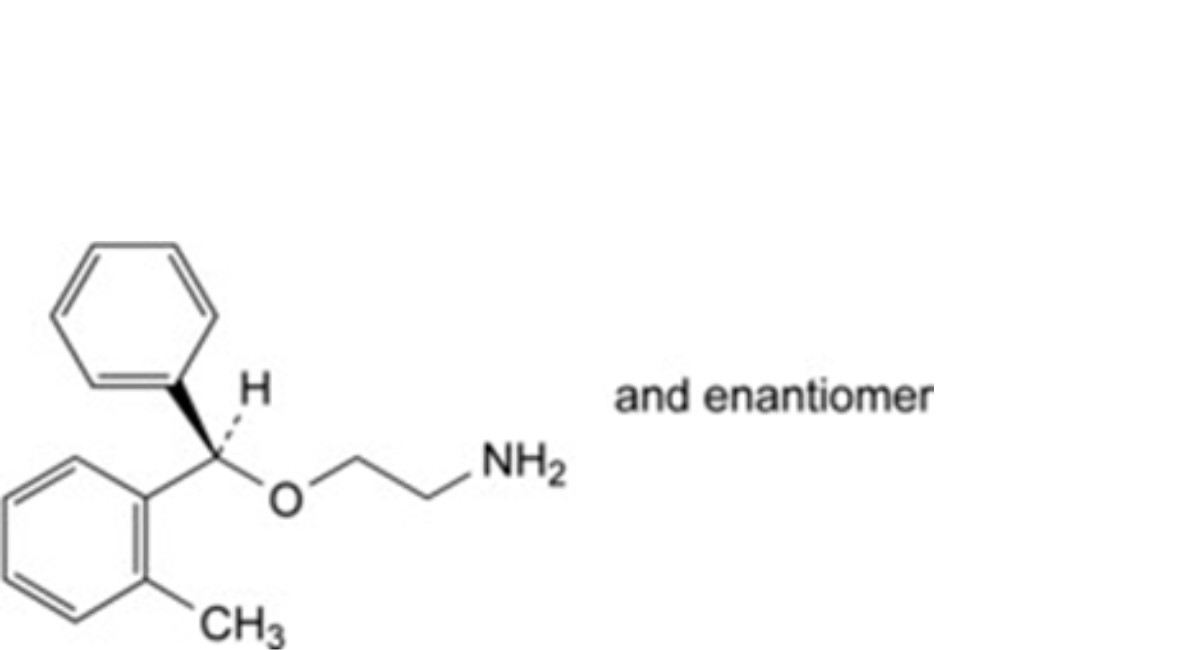
C. (RS)-2-[(2-methylphenyl)phenylmethoxy]ethanamine,
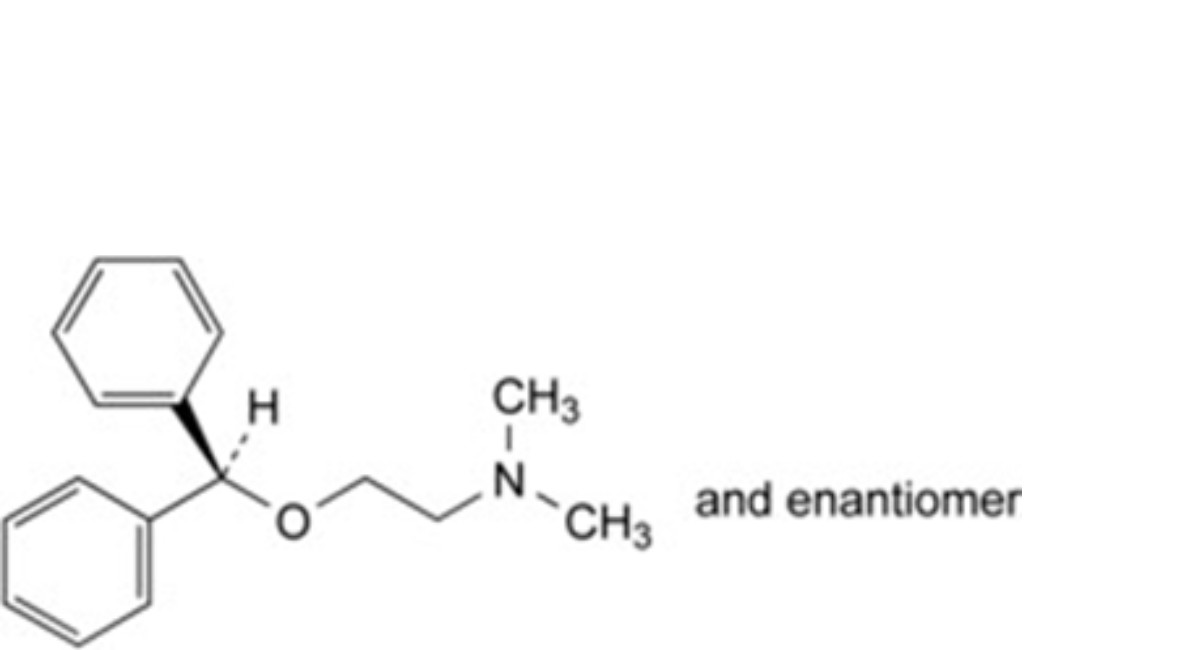
D. 2-(diphenylmethoxy)-N,N-dimethylethanamine (diphenhydramine),
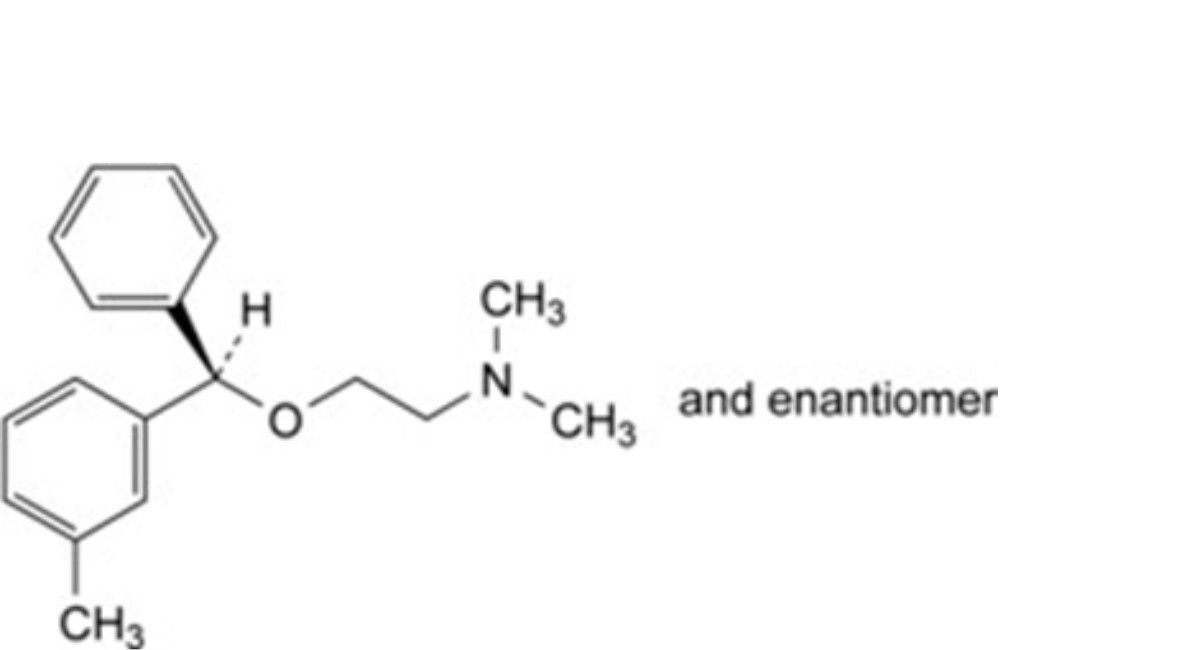
E. (RS)-N,N-dimethyl-2-[(3-methylphenyl)phenylmethoxy]ethanamine (meta-methylbenzyl isomer),
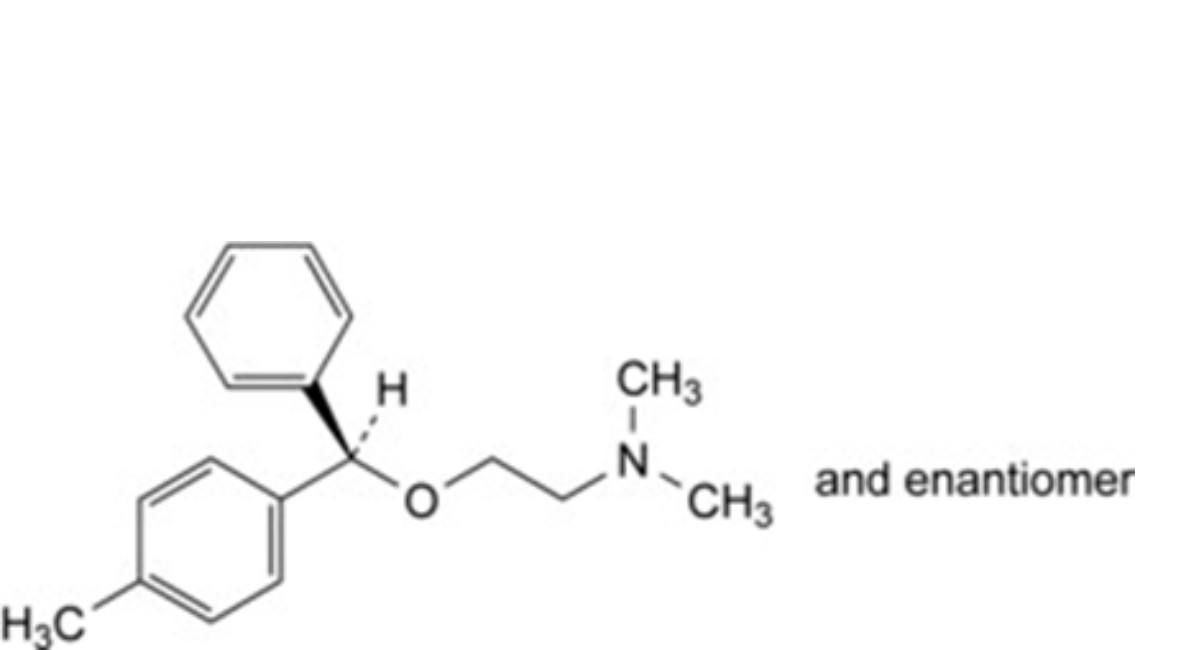
F. (RS)-N,N-dimethyl-2-[(4-methylphenyl)phenylmethoxy]ethanamine (para-methylbenzyl isomer).
Ph Eur