Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Beta2-adrenoceptor agonist; bronchodilator.
Ph Eur
DEFINITION
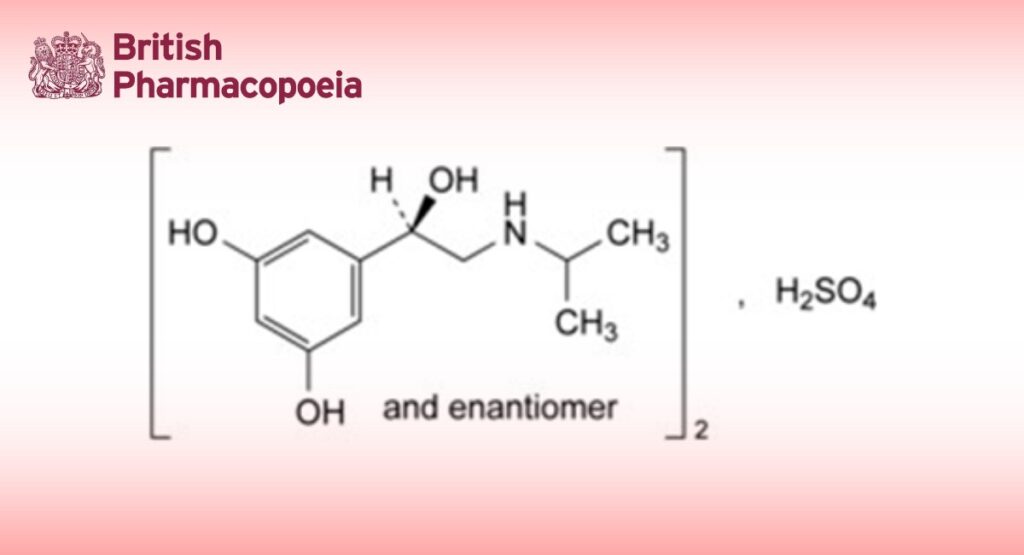
Bis[5-[(1RS)-1-hydroxy-2-[(1-methylethyl)amino]ethyl]benzene-1,3-diol] sulfate.
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, slightly hygroscopic, crystalline powder.
Solubility
Freely soluble in water, slightly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
IDENTIFICATION
First identification: B, E.
Second identification: A, C, D, E.
A. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution Dissolve 50.0 mg in a 0.04 per cent V/V solution of hydrochloric acid R and dilute to 50.0 mL with the same solution. Dilute 5.0 mL of this solution to 50.0 mL with a 0.04 per cent V/V solution of hydrochloric acid R.
Spectral range 240-350 nm.
Absorption maximum At 278 nm.
Specific absorbance at the absorption maximum 68.5 to 76.0 (anhydrous substance).
B. Infrared absorption spectrophotometry (2.2.24).
Comparison orciprenaline sulfate CRS.
If the spectra obtained show differences, dissolve separately, with heating, 50 mg of the substance to be examined and 50 mg of the reference substance, in the minimum volume of water R. Add 10 mL of acetone R and centrifuge. Dry the precipitates at 40 °C under reduced pressure for 3 h and record new spectra using the residues.
C. Thin-layer chromatography (2.2.27).
Test solution Dissolve 10 mg of the substance to be examined in ethanol (96 per cent) R and dilute to 10 mL with the same solvent.
Reference solution (a) Dissolve 10 mg of orciprenaline sulfate CRS in ethanol (96 per cent) R and dilute to 10 mL with the same solvent.
Reference solution (b) Dissolve 10 mg of orciprenaline sulfate CRS and 10 mg of salbutamol CRS in ethanol (96 per cent) R and dilute to 10 mL with the same solvent.
Plate TLC silica gel G plate R.
Mobile phase ammonia R, water R, aldehyde-free methanol R (1.5:10:90 V/V/V). Application 2 µL.
Development Over 2/3 of the plate.
Drying In air.
Detection Spray with a 10 g/L solution of potassium permanganate R. System suitability Reference solution (b):
— the chromatogram shows 2 clearly separated principal spots.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
D. Dissolve about 20 mg in 2 mL of ethanol (96 per cent) R. Add 2 mL of a 1 g/L solution of dichloroquinonechlorimide R in ethanol (96 per cent) R and 1 mL of sodium carbonate solution R. A violet colour is produced, turning to brown.
E. It gives reaction (a) of sulfates (2.3.1).
TESTS
Solution S
Dissolve 2.0 g in carbon dioxide-free water R and dilute to 20 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
pH (2.2.3)
4.0 to 5.5 for solution S.
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 20 mg of the substance to be examined in the mobile phase and dilute to 20 mL with the mobile phase.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 10.0 mL with the mobile phase.
Reference solution (b) Dissolve 2 mg of orciprenaline for system suitability CRS (containing impurities A and B) in 2.0 mL of the mobile phase.
Column:
— size: l = 0.125 m, Ø = 4.0 mm;
— stationary phase: spherical end-capped octadecylsilyl silica gel for chromatography R (5 µm);
— temperature: 45 °C.
Mobile phase Dissolve 9.1 g of potassium dihydrogen phosphate R and 4.6 g of sodium octanesulfonate R in water R, adjust to pH 4.0 with dilute phosphoric acid R and dilute to 1000 mL with water R. Add 140 mL of acetonitrile R.
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 280 nm.
Injection 10 µL.
Run time Twice the retention time of orciprenaline.
Identification of impurities Use the chromatogram supplied with orciprenaline for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A and B.
Relative retention With reference to orciprenaline (retention time = about 7 min): impurity A = about 0.9; impurity B = about 1.3.
System suitability Reference solution (b):
— resolution: minimum 2.0 between the peaks due to impurity A and orciprenaline.
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity B by 0.3;
— impurities A, B: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
— total: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Phenone
Maximum 0.1 per cent.
Dissolve 0.50 g in a 0.04 per cent V/V solution of hydrochloric acid R and dilute to 25.0 mL with the same solution. The absorbance (2.2.25) of the solution measured at 328 nm is not greater than 0.16.
Iron (2.4.9)
Maximum 20 ppm.
The residue obtained in the test for sulfated ash complies with the test. Prepare the reference solution using iron standard solution (2 ppm Fe) R.
Water (2.5.12)
Maximum 2.0 per cent, determined on 1.000 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.400 g in 5 mL of anhydrous formic acid R and add 30 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid using 0.1 mL of crystal violet solution R as indicator. 1 mL of 0.1 M perchloric acid is equivalent to 52.06 mg of C22H36N2O10S.
STORAGE
In an airtight container, protected from light.
IMPURITIES
Specified impurities A, B.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.
Control of impurities in substances for pharmaceutical use) C.
A. (4RS)-2-(1-methylethyl)-1,2,3,4-tetrahydroisoquinoline-4,6,8-triol,
B. 1-(3,5-dihydroxyphenyl)-2-[(1-methylethyl)amino]ethanone,
C. 3-hydroxy-5-[(1RS)-1-hydroxy-2-[(1-methylethyl)amino]ethyl]cyclohex-2-enone.
Ph Eur