Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Serotonin 5-HT3 antagonist; treatment of nausea and vomiting.
Preparations
Ondansetron Injection Ondansetron Tablets Ph Eur
DEFINITION
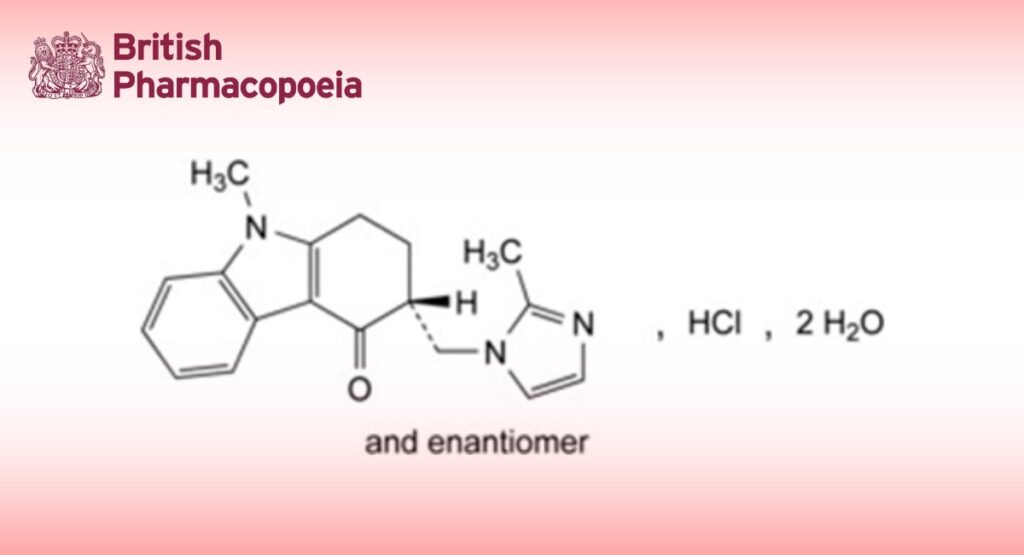
(3RS)-9-Methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one hydrochloride dihydrate.
Content
97.5 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Sparingly soluble in water, soluble in methanol, sparingly soluble in ethanol (96 per cent), slightly soluble in methylene chloride.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison ondansetron hydrochloride dihydrate CRS.
B. It gives reaction (a) of chlorides (2.3.1).
TESTS
Impurity B
Thin-layer chromatography (2.2.27).
Solvent mixture concentrated ammonia R, ethanol (96 per cent) R, methanol R (0.5:100:100 V/V/V).
Test solution Dissolve 0.125 g of the substance to be examined in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (a) Dissolve 12.5 mg of ondansetron for TLC system suitability CRS (containing impurities A and B) in the solvent mixture and dilute to 1 mL with the solvent mixture.
Reference solution (b) Dilute 1 mL of the test solution to 100 mL with the solvent mixture. Dilute 4.0 mL of this solution to 10.0 mL with the solvent mixture.
Plate TLC silica gel F254 plate R.
Mobile phase concentrated ammonia R, methanol R, ethyl acetate R, methylene chloride R (2:40:50:90 V/V/V/V).
Application 20 µL.
Development Over 3/4 of the plate.
Drying In air.
Detection Examine in ultraviolet light at 254 nm.
Retardation factors Impurity A = about 0.3; impurity B = about 0.4; ondansetron = about 0.6.
System suitability Reference solution (a):
— the chromatogram shows 3 clearly separated spots.
Limit:
— impurity B: any spot due to impurity B is not more intense than the principal spot in the chromatogram obtained with reference solution (b) (0.4 per cent).
Related substances
Liquid chromatography (2.2.29).
Test solution (a) Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Test solution (b) Dissolve 90.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase. Dilute 10.0 mL of the solution to 100.0 mL with the mobile phase.
Reference solution (a) Dilute 2.0 mL of test solution (a) to 100.0 mL with the mobile phase. Dilute 10.0 mL of this solution to 100.0 mL with the mobile phase.
Reference solution (b) Dissolve 5.0 mg of ondansetron impurity E CRS and 5 mg of ondansetron impurity A CRS in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (c) Dissolve 5 mg of ondansetron for LC system suitability CRS (containing impurities C and D) in the mobile phase and dilute to 10 mL with the mobile phase.
Reference solution (d) Dissolve 5.0 mg of ondansetron impurity D CRS in the mobile phase and dilute to 100.0 mL with the mobile phase. Dilute 1.0 mL of the solution to 100.0 mL with the mobile phase.
Reference solution (e) Dissolve 90.0 mg of ondansetron hydrochloride dihydrate CRS in the mobile phase and dilute to 100.0 mL with the mobile phase. Dilute 10.0 mL of the solution to 100.0 mL with the mobile phase.
Reference solution (f) Dissolve 5.0 mg of ondansetron impurity F CRS and 5 mg of ondansetron impurity G CRS in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (g) To 1.0 mL of reference solution (b) add 1.0 mL of reference solution (f) and dilute to 100.0 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: cyanosilyl silica gel for chromatography R (5 µm).
Mobile phase Mix 20 volumes of acetonitrile R1 and 80 volumes of a 2.8 g/L solution of sodium dihydrogen phosphate monohydrate R previously adjusted to pH 5.4 with a 40 g/L solution of sodium hydroxide R.
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 216 nm.
Injection 20 µL of test solution (a) and reference solutions (a), (b), (c), (d), (f) and (g).
Run time 1.5 times the retention time of ondansetron.
Identification of impurities:
— use the chromatogram supplied with ondansetron for LC system suitability CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities C and D;
— use the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A and E;
— use the chromatogram obtained with reference solution (f) to identify the peaks due to impurities F and G.
Relative retention With reference to ondansetron (retention time = about 18 min): impurity E = about 0.17; impurity F = about 0.20 (E and F may coelute); impurity C = about 0.35; impurity D = about 0.45; impurity A = about 0.80; impurity G = about 0.89 (A and G may coelute or be inverted).
System suitability Reference solution (c):
— resolution: minimum 2.5 between the peaks due to impurities C and D.
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity C by 0.6;
— impurity C: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
— sum of impurities A and G: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
— sum of impurities E and F: not more than the sum of the areas of the corresponding peaks in the chromatogram obtained with reference solution (g) (0.2 per cent);
— impurity D: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (d) (0.15 per cent);
— unspecified impurities: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
— total: maximum 0.4 per cent;
— disregard limit: 0.25 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Water (2.5.12)
9.0 per cent to 10.5 per cent, determined on 0.200 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Injection Test solution (b) and reference solution (e).
System suitability Reference solution (e):
— symmetry factor: maximum 2.5 for the peak due to ondansetron.
Calculate the percentage content of C18H20ClN3O taking into account the assigned content of ondansetron hydrochloride dihydrate CRS.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C, D, E, F, G.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.
Control of impurities in substances for pharmaceutical use) H.
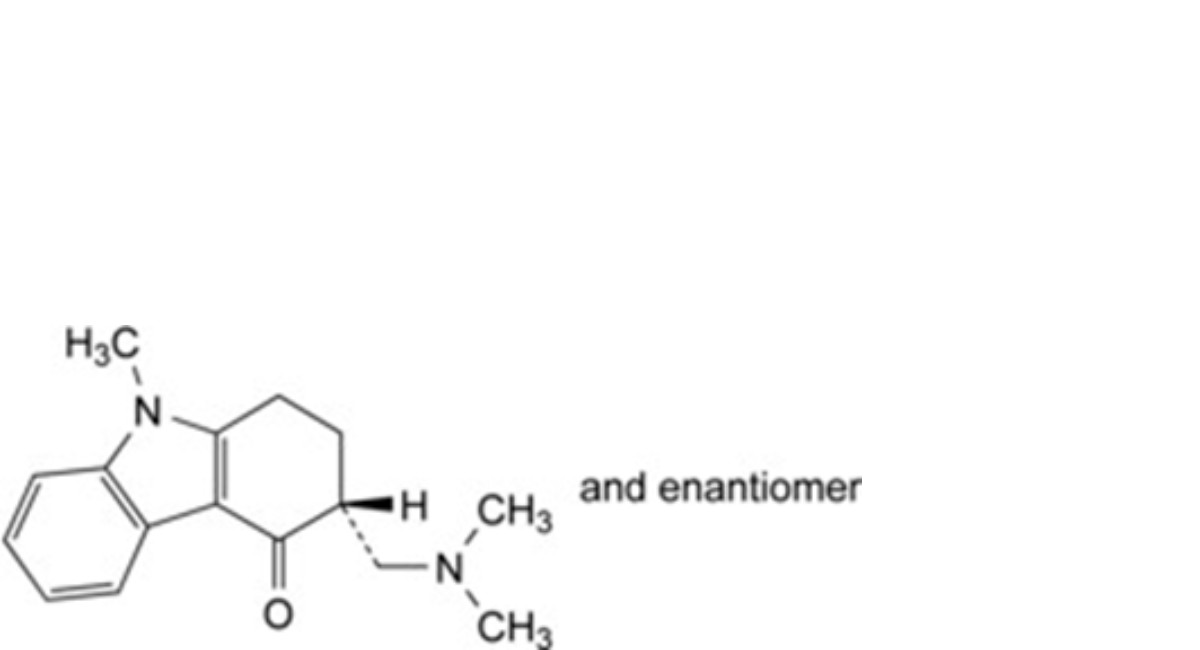
A. (3RS)-3-[(dimethylamino)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one,
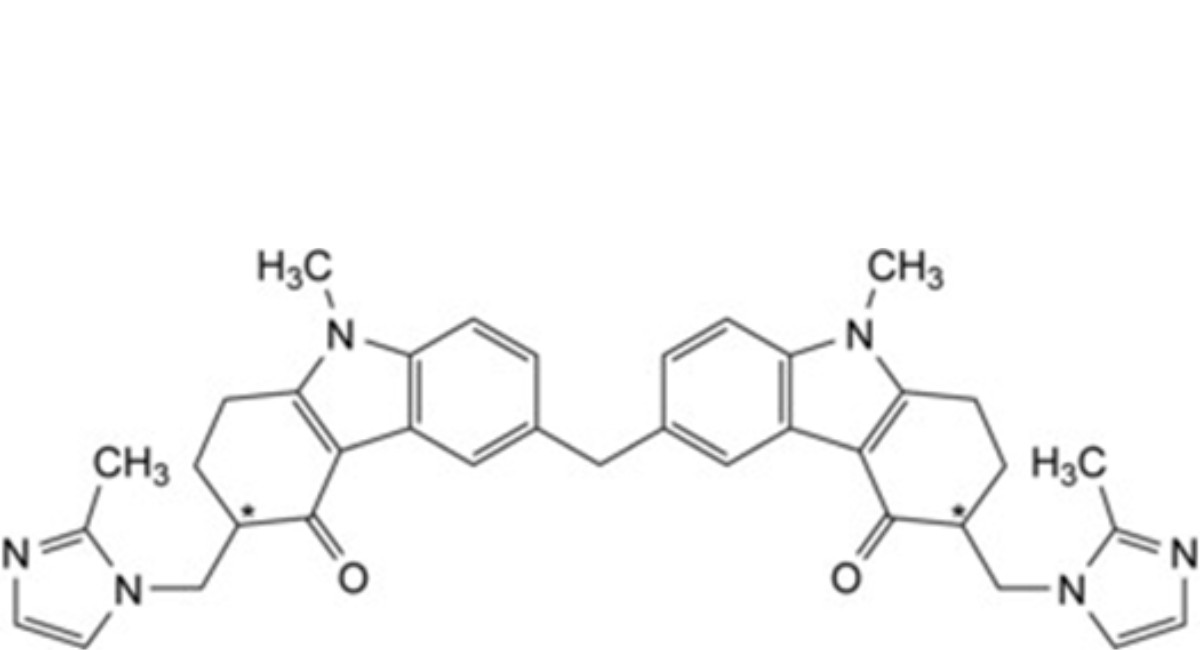
B. 6,6′-methylenebis[(3Ξ)-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol- 4-one],
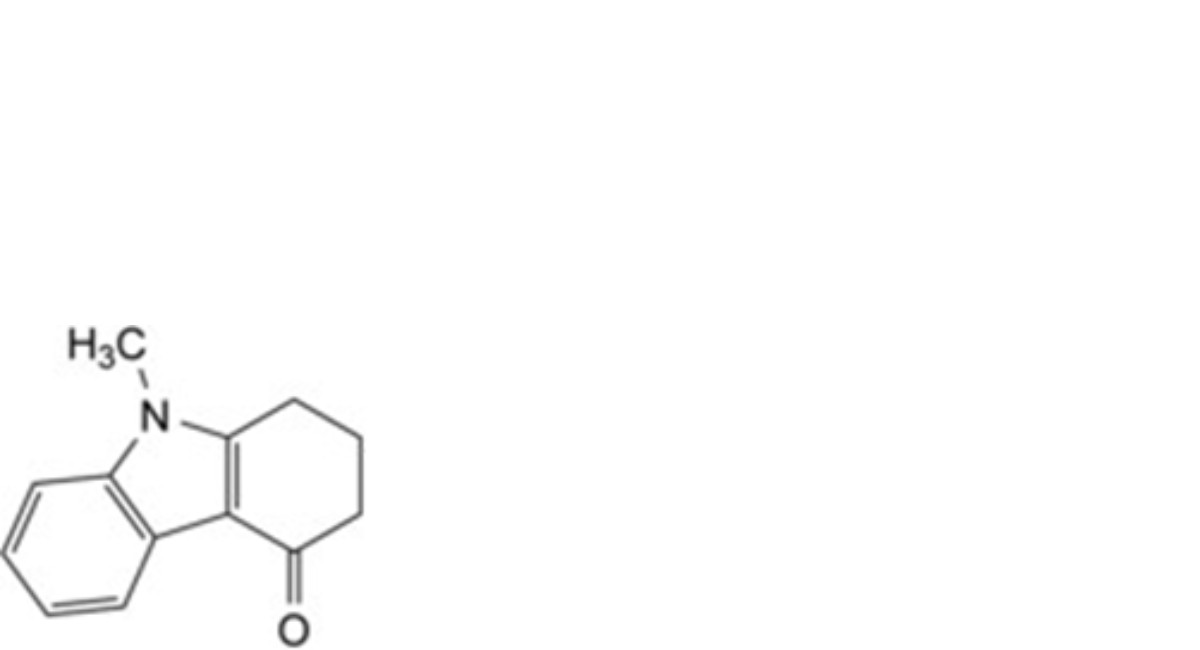
C. 9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one,
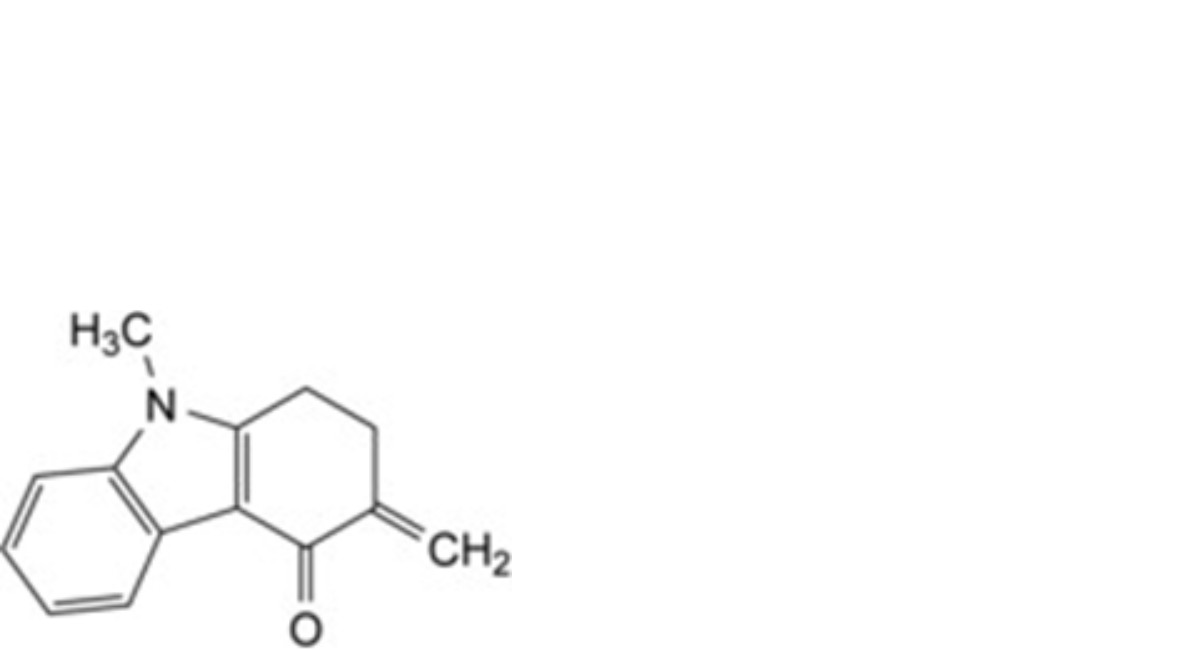
D. 9-methyl-3-methylidene-1,2,3,9-tetrahydro-4H-carbazol-4-one,

E. 1H-imidazole,

F. 2-methyl-1H-imidazole,
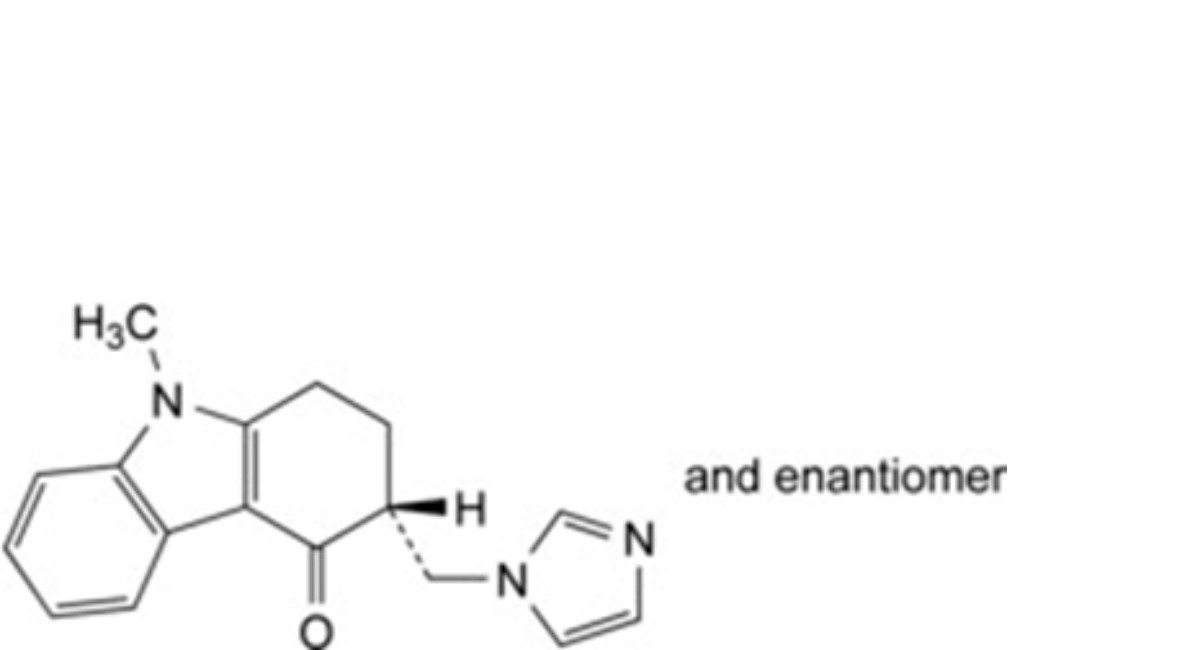
G. (3RS)-3-[(1H-imidazol-1-yl)methyl]-9-methyl-1,2,3,9-tetrahydro-4H-carbazol-4-one (C- demethylondansetron),
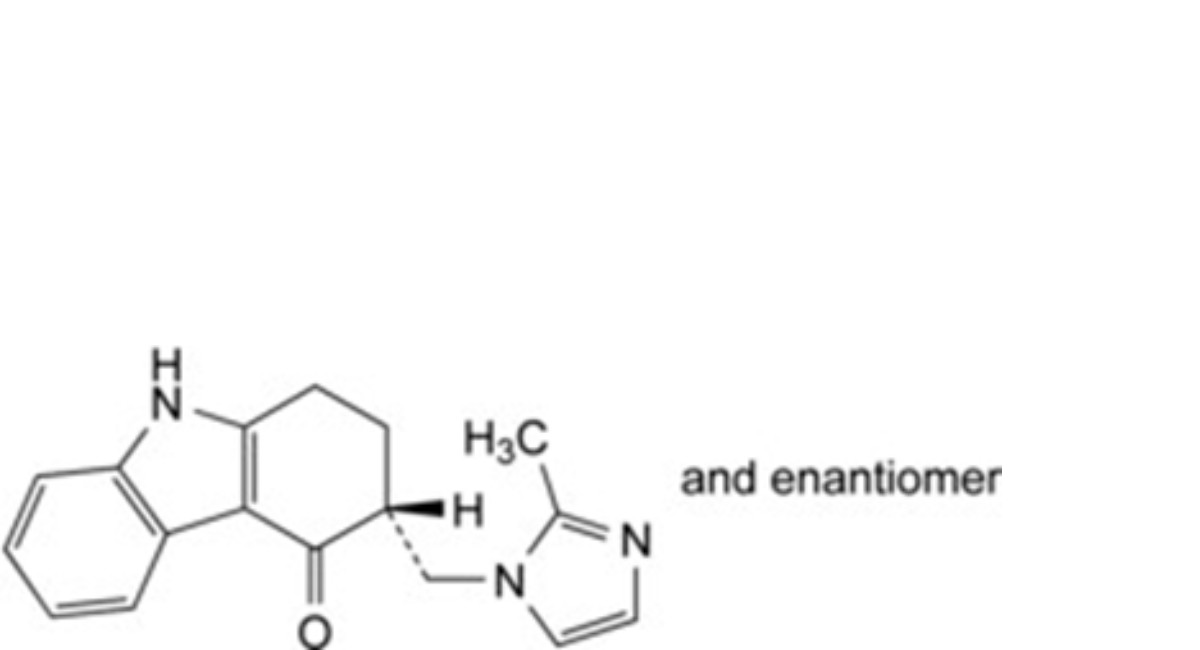
H. (3RS)-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one (N- demethylondansetron).
Ph Eur