Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Proton pump inhibitor; treatment of peptic ulcer disease.
Preparation
Omeprazole for Injection
Ph Eur
DEFINITION
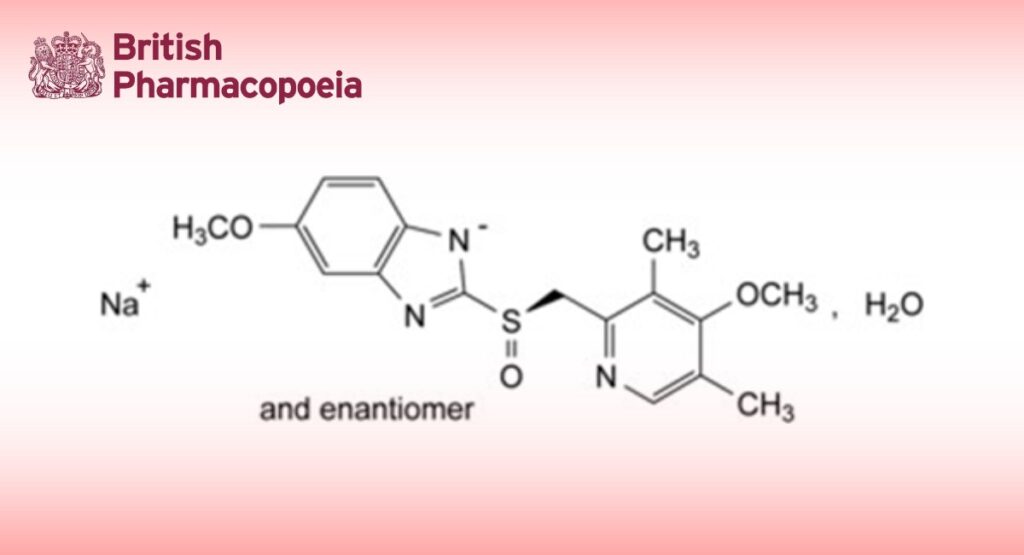
Sodium 5-methoxy-2-[(RS)-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-1H-benzimidazole monohydrate.
Content
98.0 per cent to 101.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, hygroscopic powder.
Solubility
Freely soluble in water and in ethanol (96 per cent), soluble in propylene glycol, very slightly soluble in methylene chloride.
IDENTIFICATION
A. Optical rotation (2.2.7): -0.10° to + 0.10°, determined on solution S.
B. Infrared absorption spectrophotometry (2.2.24).
Preparation Dissolve 0.50 g of the substance to be examined in 1.50 mL of water R, add 3.0 mL of
methanol R and stir; while stirring, adjust to pH 8-9 by adding, dropwise, dilute acetic acid R (about 0.4 mL); continue stirring until crystallisation and isolate the crystalline precipitate by filtration; wash with 5 mL of water R, then 2 mL of methanol R, and dry in vacuo at 40 °C for 30 min.
Comparison omeprazole CRS.
If the spectra obtained in the solid state show differences, dissolve the crystalline precipitate and the reference substance separately in methanol R, evaporate to dryness and record new spectra using the residues.
C. Ignite 1 g and cool. Add 1 mL of water R to the residue and neutralise with hydrochloric acid R. Filter and dilute the filtrate to 4 mL with water R. 0.1 mL of the solution gives reaction (b) of sodium (2.3.1).
TESTS
Solution S
Dissolve 0.50 g in carbon dioxide-free water R and dilute to 25 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution B6 (2.2.2, Method II).
pH (2.2.3)
10.3 to 11.3 for solution S.
Related substances
Liquid chromatography (2.2.29). Prepare solutions immediately before use.
Test solution Dissolve 3 mg of the substance to be examined in the mobile phase and dilute to 25.0 mL with the mobile phase.
Reference solution (a) Dissolve 1 mg of omeprazole CRS and 1 mg of omeprazole impurity D CRS in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 10.0 mL with the mobile phase.
Reference solution (c) Dissolve 3 mg of omeprazole for peak identification CRS (containing impurity E) in the mobile phase and dilute to 25.0 mL with the mobile phase.
Column:
— size: l = 0.125 m, Ø = 4.6 mm;
— stationary phase: octylsilyl silica gel for chromatography R (5 µm).
Mobile phase Mix 27 volumes of acetonitrile R and 73 volumes of a 1.4 g/L solution of disodium hydrogen phosphate dodecahydrate R, previously adjusted to pH 7.6 with phosphoric acid R.
Flow rate 1 mL/min.
Detection Spectrophotometer at 280 nm.
Injection 40 µL.
Run time 5 times the retention time of omeprazole.
Identification of impurities Use the chromatogram supplied with omeprazole for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peak due to impurity E; use the chromatogram obtained with reference solution (a) to identify the peak due to impurity D.
Relative retention With reference to omeprazole (retention time = about 9 min): impurity E = about 0.6; impurity D = about 0.8.
System suitability Reference solution (a):
— resolution: minimum 3.0 between the peaks due to impurity D and omeprazole; if necessary adjust the pH of the aqueous part of the mobile phase or the concentration of acetonitrile R; an increase in the pH will improve the resolution.
Limits:
— impurities D, E: for each impurity, not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.15 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— total: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Water (2.5.12)
4.5 per cent to 10.0 per cent, determined on 0.300 g.
ASSAY
Dissolve 0.300 g in 50 mL of water R. Titrate with 0.1 M hydrochloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M hydrochloric acid corresponds to 36.74 mg of C17H18N3NaO3S.
STORAGE
In an airtight container, protected from light.
IMPURITIES
Specified impurities D, E.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.
Control of impurities in substances for pharmaceutical use) A, B, C.
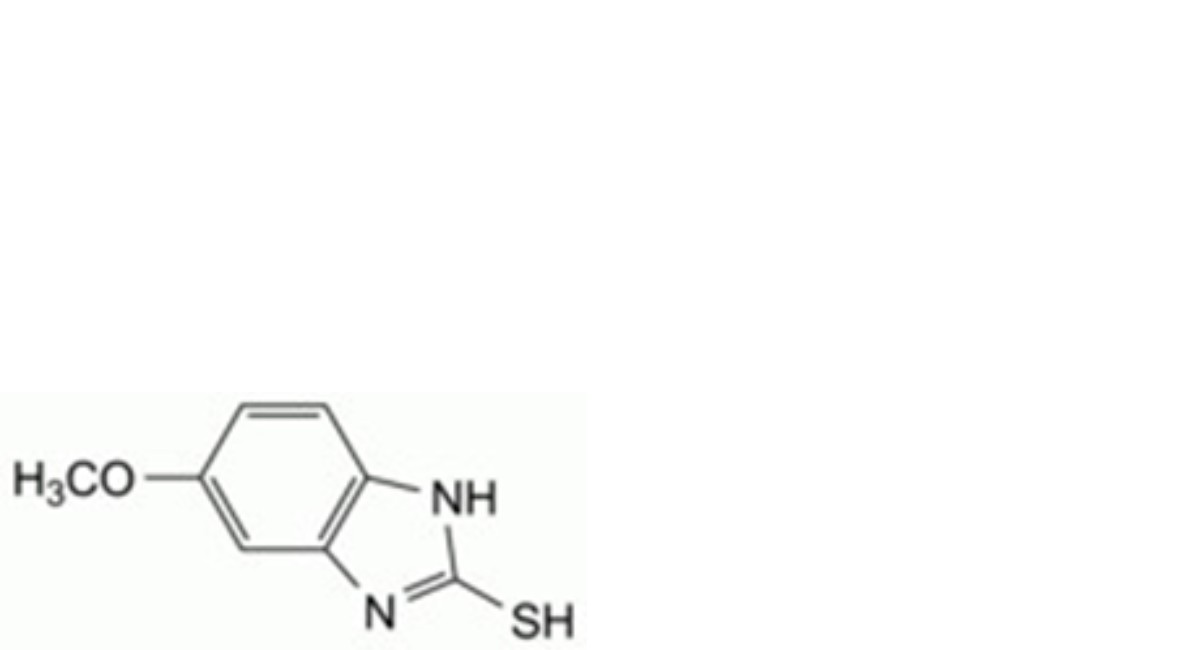
A. 5-methoxy-1H-benzimidazole-2-thiol,
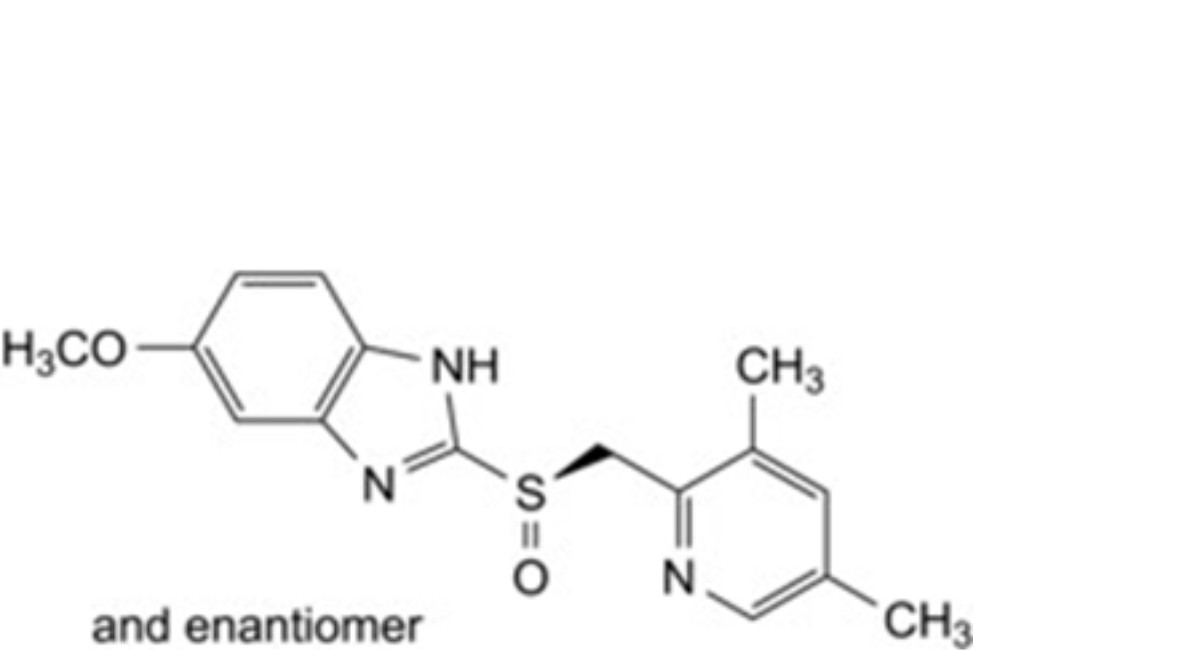
B. 2-[(RS)-[(3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-5-methoxy-1H-benzimidazole,
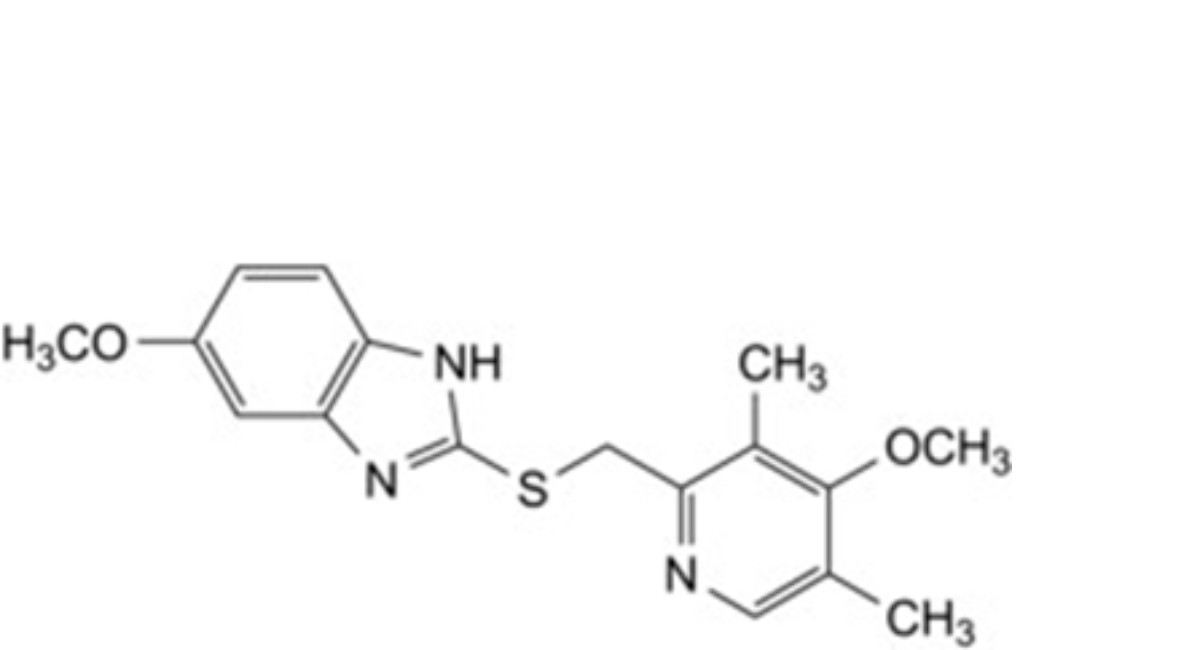
C. 5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfanyl]-1H-benzimidazole (ufiprazole),
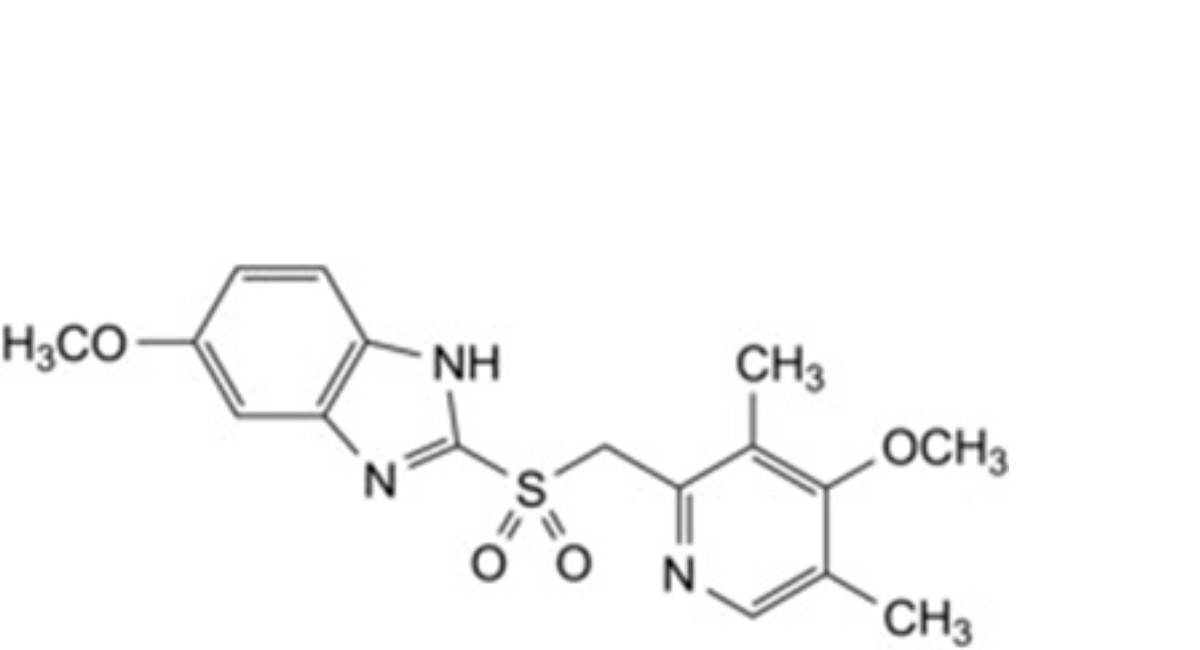
D. 5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfonyl]-1H-benzimidazole (omeprazole- sulfone),