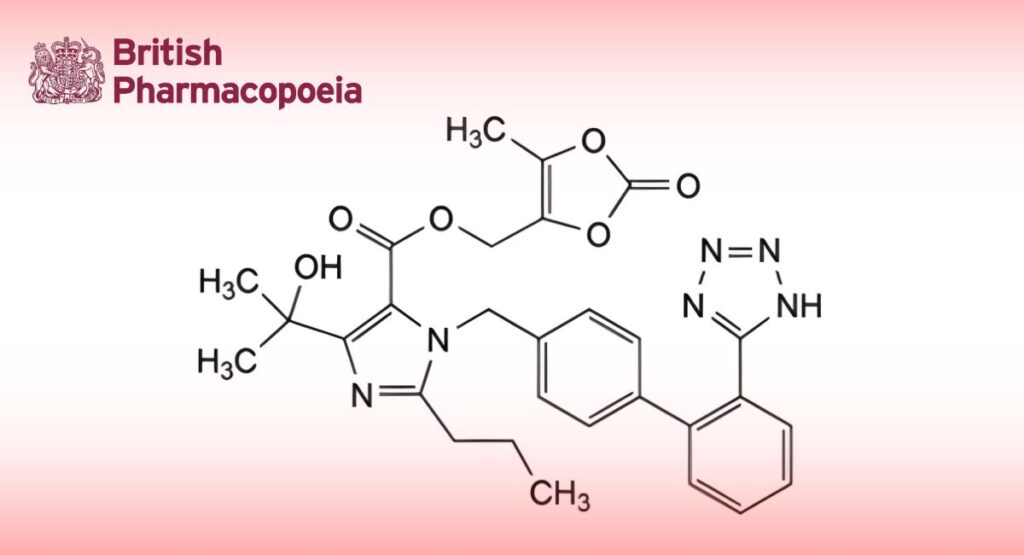
(Ph. Eur. monograph 2600)
C29H30N6O6 558.6 144689-63-4
Action and use
Angiotensin II (AT1) receptor antagonist.
Preparation
Olmesartan Tablets
DEFINITION
(5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-imidazole-5-carboxylate.
Content
97.5 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, slightly soluble in ethanol (96 per cent), practically insoluble in heptane.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison: olmesartan medoxomil CRS.
TESTS
Related substances
Liquid chromatography (2.2.29).
Test solution (a): Dissolve 25 mg of the substance to be examined in acetonitrile R and dilute to 25.0 mL with the same solvent.
Test solution (b): Dissolve 25.0 mg of the substance to be examined in acetonitrile R and dilute to 50.0 mL with the same solvent.
Reference solution (a): Dissolve 5 mg of olmesartan medoxomil for system suitability CRS (containing impurities A, B and C) in acetonitrile R and dilute to 5 mL with the same solvent.
Reference solution (b): Dilute 1.0 mL of test solution (a) to 50.0 mL with acetonitrile R. Dilute 1.0 mL of this solution to 10.0 mL with acetonitrile R.
Reference solution (c): Dissolve 25.0 mg of olmesartan medoxomil CRS in acetonitrile R and dilute to 50.0 mL with the same solvent.
Column:
— size: l = 0.10 m, Ø = 4.6 mm;
— stationary phase: end-capped octylsilyl silica gel for chromatography R (3.5 μm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: mix 20 volumes of acetonitrile R and 80 volumes of a 2.04 g/L solution of potassium dihydrogen phosphate R previously adjusted to pH 3.4 with a 1.73 g/L solution of phosphoric acid R;
— mobile phase B: mix 20 volumes of a 2.04 g/L solution of potassium dihydrogen phosphate R, previously adjusted to pH 3.4 with a 1.73 g/L solution of phosphoric acid R, and 80 volumes of acetonitrile R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 10 | 75 | 25 |
| 10 – 35 | 75 → 0 | 25 → 100 |
| 35 – 45 | 0 | 100 |
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 250 nm.
Injection: 10 μL of test solution (a) and reference solutions (a) and (b).
Identification of impurities: Use the chromatogram supplied with olmesartan medoxomil for system suitability CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to
impurities A, B and C.
Relative retention: With reference to olmesartan medoxomil (retention time = about 10 min): impurity A = about 0.2; impurity B = about 0.7; impurity C = about 1.5.
System suitability: Reference solution (a):
— resolution: minimum 3.5 between the peaks due to impurity B and olmesartan medoxomil.
Limits:
— impurity A: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (b) (0.4 per cent);
— impurity C: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.3 per cent);
— unspecified impurities: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— total: not more than 3.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.7 per cent);
— disregard limit: 0.25 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Acetone
Head-space gas chromatography (2.2.28): use the direct calibration method.
Internal standard solution: Dilute 1.0 mL of butanol R to 100.0 mL with dimethyl sulfoxide R.
Test solution: Dissolve 0.250 g of the substance to be examined in dimethyl sulfoxide R, add 2.0 mL of the internal standard solution and dilute to 10.0 mL with dimethyl sulfoxide R.
Reference solution: Dilute 0.50 mL of acetone R to 200.0 mL with dimethyl sulfoxide R. Dilute 15.0 mL of the solution to 100.0 mL with dimethyl sulfoxide R. To 25.0 mL of this solution add 10.0 mL of the internal
standard solution and dilute to 50.0 mL with dimethyl sulfoxide R.
Column:
— material: fused silica;
— size: l = 30 m, Ø = 0.53 mm;
— stationary phase: macrogol 20 000 R (film thickness 1 μm).
Carrier: gas nitrogen for chromatography R or helium for chromatography R.
Flow rate: 4.0 mL/min.
Split ratio: 1:5.
Static head-space conditions that may be used:
— equilibration temperature: 80 °C;
— equilibration time: 30 min.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 5 | 50 |
| 5 – 18 | 50 → 180 | |
| 18 – 23 | 180 | |
| Injection port | 200 | |
| Detection | 200 |
Detection: Flame ionisation.
Injection: 1 mL.
Calculate the content of acetone, taking its relative density to be 0.79 at 20 °C.
Limit:
— acetone: maximum 0.6 per cent.
Water (2.5.32)
Maximum 0.5 per cent, determined on 0.500 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Mobile phase: Mobile phase B, mobile phase A (25:75 V/V).
Injection: Test solution (b) and reference solution (c).
Retention time: Olmesartan medoxomil = about 10 min.
Run time: 1.5 times the retention time of olmesartan medoxomil.
Calculate the percentage content of C29H30N6O6 taking into account the assigned content of olmesartan medoxomil CRS.
IMPURITIES
Specified impurities A, C.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for
other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.
Control of impurities in substances for pharmaceutical use) B, D.
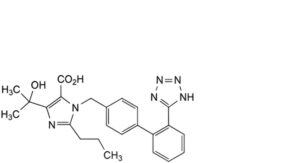
A. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-imidazole-5-carboxylic acid (olmesartan),
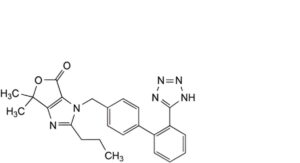
B. 6,6-dimethyl-2-propyl-3-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-3,6-dihydro-4H-furo[3,4-d]imidazol-4-one,
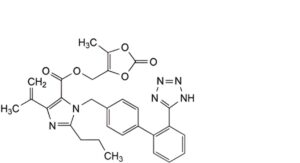
C. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(1-methylethenyl)-2-propyl-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-imidazole-5-carboxylate,
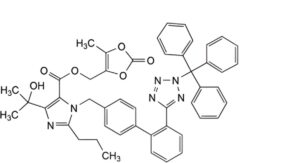
D. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2′-[(2-triphenylmethyl)-2H-tetrazol-5-yl]biphenyl-4-yl]methyl]-1H-imidazole-5-carboxylate.