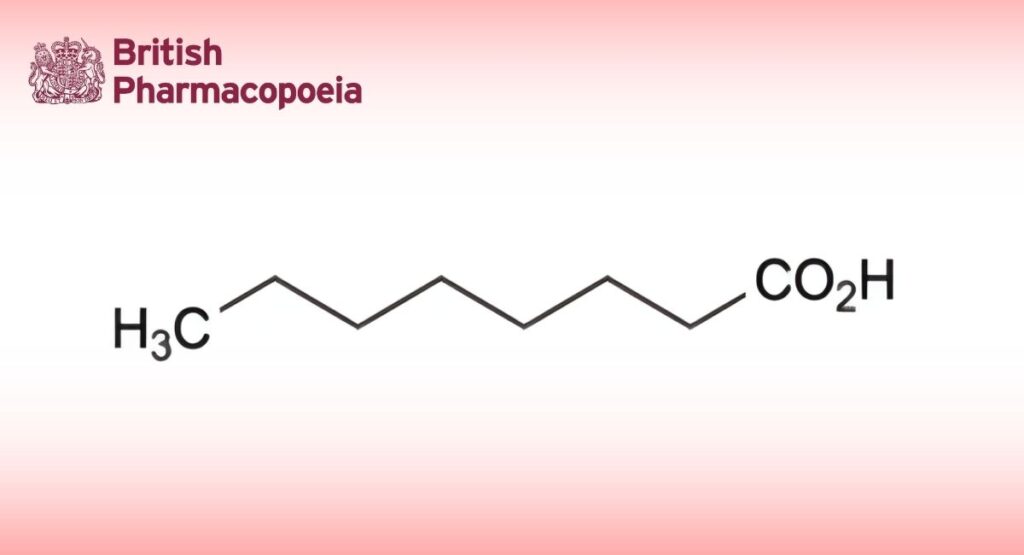
(Caprylic Acid, Ph. Eur. monograph 1401)
C8H16O2 144.2 124-07-2
Action and use
Excipient.
DEFINITION
Octanoic acid.
Content
99.0 per cent to 100.5 per cent (anhydrous substance).
CHARACTERS
Appearance
Clear, colourless or slightly yellowish, oily liquid.
Solubility
Very slightly soluble in water, very soluble in acetone and in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides.
IDENTIFICATION
A. Relative density (see Tests).
B. Examine the chromatograms obtained in the test for related substances.
Results: The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
TESTS
Appearance
The substance to be examined is clear (2.2.1) and not more intensely coloured than reference solution Y5 (2.2.2, Method II).
Relative density (2.2.5)
0.909 to 0.912.
Related substances
Gas chromatography (2.2.28): use the normalisation procedure.
Test solution: Dissolve 0.10 g of the substance to be examined in ethyl acetate R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 0.10 g of caprylic acid CRS in ethyl acetate R and dilute to 10.0 mL with the same solvent.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with ethyl acetate R. Dilute 5.0 mL of this solution to 50.0 mL with ethyl acetate R.
Column:
— material: fused silica;
— size: l = 30 m, Ø = 0.25 mm;
— stationary phase: macrogol 20 000 2-nitroterephthalate R (film thickness 0.25 μm).
Carrier gas helium for chromatography R.
Flow rate: 1.5 mL/min.
Split ratio: 1:100.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 0 – 1 | 100 |
| 1 – 25 | 100 → 220 | |
| 25 – 35 | 220 | |
| Injection port | 250 | |
| Detector | 250 |
Detection: Flame ionisation.
Injection: 1 μL.
System suitability: Reference solution (b):
— signal-to-noise ratio: minimum 5 for the principal peak.
Limits:
— any impurity: for each impurity, maximum 0.3 per cent;
— total: maximum 0.5 per cent;
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Water (2.5.12)
Maximum 0.7 per cent, determined on 1.000 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.125 g in 25 mL of ethanol (96 per cent) R. Titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M sodium hydroxide is equivalent to 14.42 mg of C8H16O2.
IMPURITIES
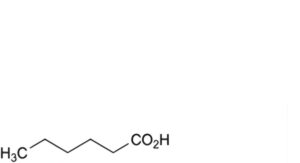
A. hexanoic acid,
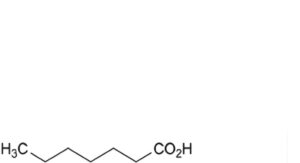
B. heptanoic acid,
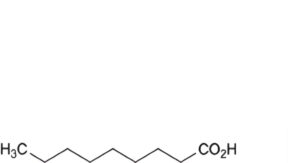
C. nonanoic acid,
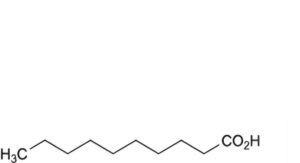
D. decanoic acid,
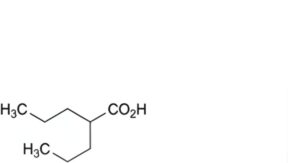
E. 2-propylpentanoic acid (valproic acid),
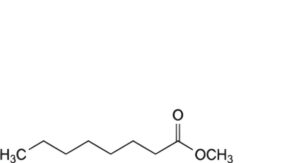
F. methyl octanoate,
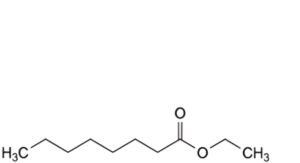
G. ethyl octanoate,
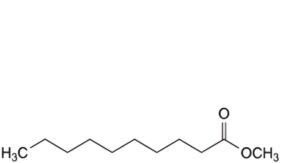
H. methyl decanoate,
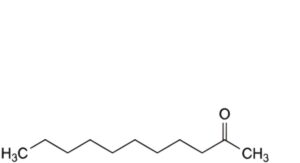
I. undecan-2-one,
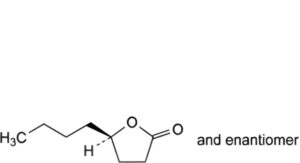
J. 5-butyltetrahydrofuran-2-one (γ-hydroxyoctanoic acid lactone).