Nizatidine Intravenous Infusion
Action and use
Histamine H2 receptor antagonist; treatment of peptic ulcer.
DEFINITION
Nizatidine Infusion is a sterile solution containing Nizatidine. It is prepared by diluting Nizatidine Sterile Concentrate with a suitable diluent in accordance with the manufacturer’s instructions.
The infusion complies with the requirements stated under Parenteral Preparations.
NIZATIDINE STERILE CONCENTRATE
DEFINITION
Nizatidine Sterile Concentrate is a sterile solution of Nizatidine in Water for Injections.
The concentrate complies with the requirements for Concentrates for Injections or Infusions stated under Parenteral Preparations and with the following requirements.
Content of nizatidine, C12H21N5O2S2
95.0 to 105.0% of the stated amount.
CHARACTERISTICS
A clear, colourless to yellow solution that darkens on storage.
IDENTIFICATION
Shake a volume of the concentrate containing 50 mg of Nizatidine with 10 mL of dichloromethane for 5 minutes. Filter the dichloromethane layer through anhydrous sodium sulfate and evaporate the filtrate to dryness on a rotary evaporator at
40°. Cool the residue in a refrigerator for 15 hours and, if necessary, induce crystallisation by scratching the wall of the container with a glass rod. The infrared absorption spectrum of the dried residue, Appendix II A, is concordant with the
reference spectrum of nizatidine (RS 410).
TESTS
Acidity or alkalinity
pH of the concentrate diluted with water to contain 1.0% w/v of Nizatidine, 6.0 to 8.0, Appendix V L.
Colour of solution
Dilute the concentrate with water to produce a solution containing 1.0% w/v of Nizatidine. The absorbance of the solution at 410 nm, Appendix II B, is not more than 0.40 determined using water in the reference cell.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute the concentrate with mobile phase A to produce a solution containing 0.5% w/v of Nizatidine.
(2) Dilute 1 volume of solution (1) to 100 volumes with mobile phase A.
(3) 0.005% w/v of nizatidine EPCRS and 0.005% w/v of nizatidine impurity F EPCRS in mobile phase A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Supelco LC-18-DB and Kromasil C18 are suitable).
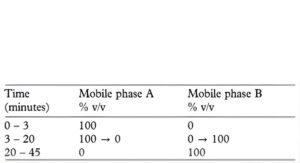
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 254 nm.
(f) Inject 20 μL of each solution.
Mobile phase A: Dissolve 5.9 g of ammonium acetate in 760 mL of water, add 1 mL of diethylamine and adjust the pH to 7.5 with 6M acetic acid (solution A). Prepare a mixture of 24 volumes of methanol and 76 volumes of solution A.
Mobile phase B: 50 volumes of methanol and 50 volumes of solution A.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2), the retention time of Nizatidine is between 10 and 20 minutes and the symmetry factor of the peak due to Nizatidine is not greater than 2.0.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peak due to Nizatidine (first peak) and Nizatidine impurity F (second peak) is at least 2.0.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak with a relative retention time of about 0.7 (impurity E) is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (2%);
the area of any peak corresponding to impurity F is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (1%);
the area of any other secondary peak is not greater than 0.3 times the area of the principal peak in the chromatogram obtained with solution (2) (0.3%);
the sum of the areas of all the secondary peaks is not greater than 3.5 times the area of the principal peak in the chromatogram obtained with solution (2) (3.5%).
Disregard any peak with an area less than 0.05 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute the concentrate with mobile phase A to produce a solution containing 0.03% w/v of Nizatidine.
(2) 0.03% w/v solution of nizatidine EPCRS in mobile phase A.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2), the symmetry factor of the peak due to Nizatidine is not greater than 2.0.
DETERMINATION OF CONTENT
Calculate the content of C12H21N5O2S2 in the concentrate using the declared content of C12H21N5O2S2 in nizatidine EPCRS.
STORAGE
Nizatidine Sterile Concentrate should be protected from light.






