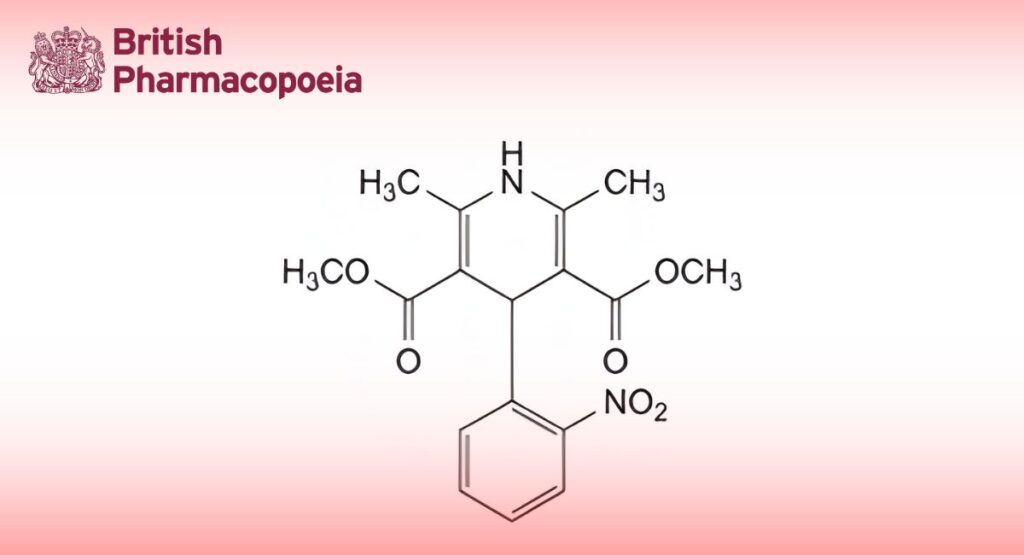
(Ph. Eur. monograph 0627)
C17H18N2O6 346.3 21829-25-4
Action and use
Calcium channel blocker.
Preparations
Nifedipine Capsules
Nifedipine Oral Suspension
Nifedipine Prolonged-release Capsules
Nifedipine Prolonged-release Capsules
Nifedipine Prolonged-release Tablets
DEFINITION
Dimethyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate.
Content
98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
Yellow, crystalline powder.
Solubility
Practically insoluble in water, freely soluble in acetone, sparingly soluble in anhydrous ethanol.
When exposed to daylight and to artificial light of certain wavelengths, it readily converts to a nitrosophenylpyridine derivative. Exposure to ultraviolet light leads to the formation of a nitrophenylpyridine derivative.
Prepare solutions immediately before use in the dark or under long-wavelength light (> 420 nm) and protect them from light.
IDENTIFICATION
First identification: B.
Second identification: A, C, D.
A. Melting point (2.2.14): 171 °C to 175 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: nifedipine CRS.
C. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in methanol R and dilute to 10 mL with the same solvent.
Reference solution: Dissolve 10 mg of nifedipine CRS in methanol R and dilute to 10 mL with the same solvent.
Plate: TLC silica gel F254 plate R.
Mobile phase: ethyl acetate R, cyclohexane R (40:60 V/V).
Application: 5 μL.
Development: Over 3/4 of the plate.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, appearance at 254 nm and size to the principal spot in the chromatogram obtained with the reference solution.
D. To 25 mg in a test tube, add 10 mL of a mixture of 1.5 volumes of hydrochloric acid R, 3.5 volumes of water R and 5 volumes of alcohol R and dissolve with gentle heating. Add 0.5 g of zinc R in granules and allow to stand for 5 min with occasional swirling. Filter into a second test tube, add 5 mL of a 10 g/L solution of sodium nitrite R to the filtrate and allow to stand for 2 min. Add 2 mL of a 50 g/L solution of ammonium sulfamate R, shake vigorously with care and add 2 mL of a 5 g/L solution of naphthylethylenediamine dihydrochloride R. An intense red colour develops which persists for not less than 5 min.
TESTS
Impurity D and other basic impurities
Transfer 4 g to a 250 mL conical flask and dissolve in 160 mL of glacial acetic acid R using an ultrasonic bath. Titrate with 0.1 M perchloric acid using 0.25 mL of naphtholbenzein solution R as indicator until the colour changes from brownish-yellow to green. Not more than 0.48 mL of 0.1 M perchloric acid is required
(0.14 per cent).
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 0.200 g of the substance to be examined in 20 mL of methanol R and dilute to 50.0 mL with the mobile phase.
Reference solution (a): Dissolve 10 mg of nifedipine impurity A CRS in methanol R and dilute to 25.0 mL with the same solvent.
Reference solution (b): Dissolve 10 mg of nifedipine impurity B CRS in methanol R and dilute to 25.0 mL with the same solvent.
Reference solution (c): Mix 1.0 mL of reference solution (a), 1.0 mL of reference solution (b) and 0.1 mL of the test solution and dilute to 20.0 mL with the mobile phase. Dilute 2.0 mL of this solution to 10.0 mL with the mobile phase.
Column:
— size: l = 0.15 m, Ø = 4.6 mm,
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: acetonitrile R, methanol R, water R (9:36:55 V/V/V).
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 235 nm.
Injection: 20 μL; inject the test solution and reference solution (c).
Run time: Twice the retention time of nifedipine.
Elution order: Impurity A, impurity B, nifedipine.
Retention time: Nifedipine = about 15.5 min.
System suitability: Reference solution (c):
— resolution: minimum 1.5 between the peaks due to impurity A and impurity B and minimum 1.5 between the peaks due to impurity B and nifedipine.
Limits:
— impurity A: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (c) (0.1 per cent),
— impurity B: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (c) (0.1 per cent),
— any other impurity: not more than the area of the peak due to nifedipine in the chromatogram obtained with reference solution (c) (0.1 per cent),
— total: not more than 0.3 per cent,
— disregard limit: 0.1 times the area of the peak due to nifedipine in the chromatogram obtained with reference solution (c) (0.01 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 2 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.1300 g in a mixture of 25 mL of 2-methyl-2-propanol R and 25 mL of perchloric acid solution R. Titrate with 0.1 M cerium sulfate using 0.1 mL of ferroin R as indicator, until the pink colour disappears. Titrate slowly towards the end of the titration. Carry out a blank titration.
1 mL of 0.1 M cerium sulfate is equivalent to 17.32 mg of C17H18N2O6.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C, D.
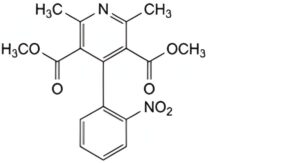
A. dimethyl 2,6-dimethyl-4-(2-nitrophenyl)pyridine-3,5-dicarboxylate (nitrophenylpyridine analogue),
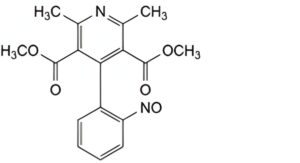
B. dimethyl 2,6-dimethyl-4-(2-nitrosophenyl)pyridine-3,5-dicarboxylate (nitrosophenylpyridine analogue),
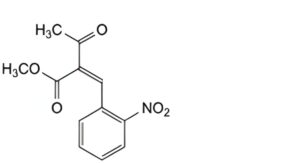
C. methyl 2-(2-nitrobenzylidene)-3-oxobutanoate,
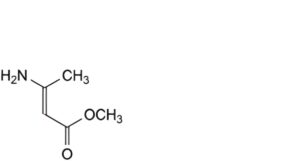
D. methyl 3-aminobut-2-enoate.