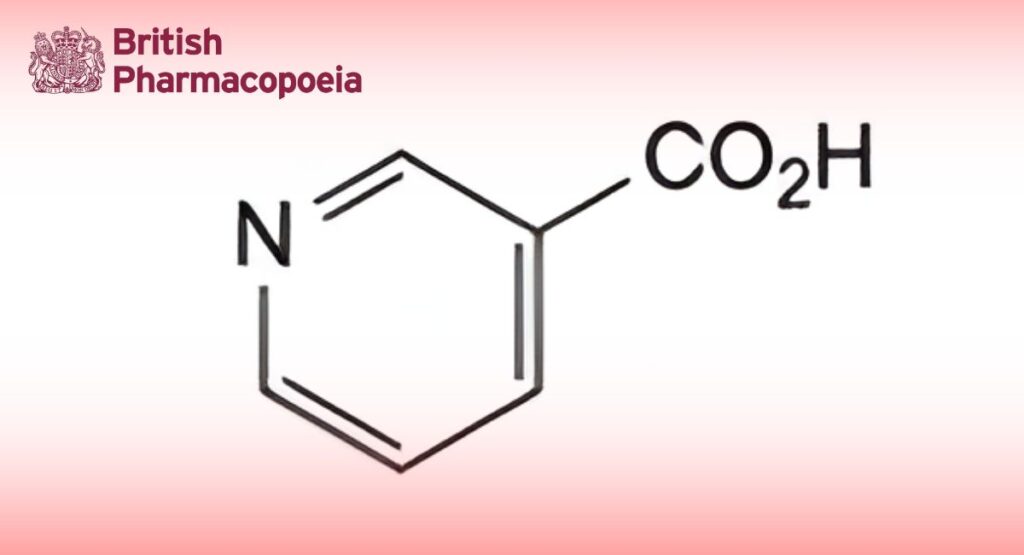
(Ph. Eur. monograph 0459)
C6H5NO2 123.1 59-67-6
Action and use
Component of vitamin B.
Preparation
Nicotinic Acid Tablets
DEFINITION
Pyridine-3-carboxylic acid.
Content
99.5 per cent to 100.5 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Sparingly soluble in water, soluble in boiling water and in boiling ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides and carbonates.
IDENTIFICATION
First identification: A, B.
Second identification: A, C.
A. Melting point (2.2.14): 234 °C to 240 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: nicotinic acid CRS.
C. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Solvent mixture: Dissolve 6.8 g of potassium dihydrogen phosphate R in 900 mL of water R, adjust to pH 7.0 with dilute sodium hydroxide solution R and dilute to 1000 mL with water R.
Test solution: Dissolve 50 mg in the solvent mixture and dilute to 100.0 mL with the solvent mixture. Dilute 1.0 mL of the solution to 25.0 mL with the solvent mixture.
Spectral range: 237-262 nm.
Absorption maximum: At 262 nm.
Absorption minimum: At 237 nm.
Absorbance ratio: A237/A262 = 0.46 to 0.50.
TESTS
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 0.120 g of the substance to be examined in 200 μL of dilute ammonia R1 and dilute to 10.0 mL with mobile phase A.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with mobile phase A. Dilute 1.0 mL of this solution to 10.0 mL with mobile phase A.
Reference solution (b): Dissolve the contents of a vial of nicotinic acid impurity mixture CRS (impurities A and B) in 1.0 mL of mobile phase A.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography compatible with 100 per cent aqueous mobile phases R (4 μm);
— temperature: 15 °C.
Mobile phase:
— mobile phase A: dilute 2 mL of acetic acid R in 950 mL of water for chromatography R, adjust to pH 5.6 with dilute ammonia R1 and dilute to 1000 mL with water for chromatography R;
— mobile phase B: acetonitrile R, methanol R (50:50 V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 10 | 100 | 0 |
| 10 – 30 | 100→20 | 0→80 |
| 30 – 35 | 20 | 80 |
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 250 nm.
Injection: 10 μL.
Identification of impurities: Use the chromatogram supplied with nicotinic acid impurity mixture CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A and B.
Relative retention: With reference to nicotinic acid (retention time = about 6 min): impurity A = about 2.7; impurity B = about 2.8.
System suitability: Reference solution (b):
— resolution: minimum 1.5 between the peaks due to impurities A and B.
Limits:
— unspecified impurities: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent);
— total: not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent);
— disregard limit: 0.3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.03 per cent).
Chlorides (2.4.4)
Maximum 200 ppm.
Dissolve 0.25 g in water R, heating on a water-bath, and dilute to 15 mL with the same solvent.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 1 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.250 g in 50 mL of water R. Add 0.25 mL of phenolphthalein solution R. Titrate with 0.1 M sodium hydroxide until a pink colour is obtained. Carry out a blank titration.
1 mL of 0.1 M sodium hydroxide is equivalent to 12.31 mg of C6H5NO2.
STORAGE
Protected from light.
IMPURITIES
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B, C, D, E, F, G, H, I.
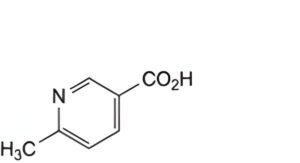
A. 6-methylpyridine-3-carboxylic acid (6-methylnicotinic acid),
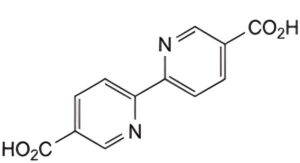
B. 2,2′-bipyridine-5,5′-dicarboxylic acid (6,6′-dinicotinic acid),
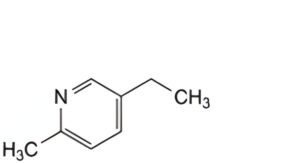
C. 5-ethyl-2-methylpyridine,
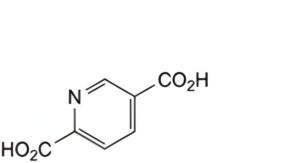
D. pyridine-2,5-dicarboxylic acid,
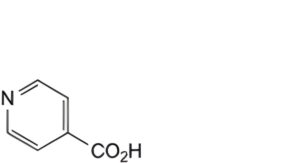
E. pyridine-4-carboxylic acid (isonicotinic acid),
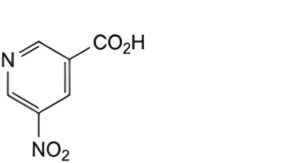
F. 5-nitropyridine-3-carboxylic acid (5-nitronicotinic acid),
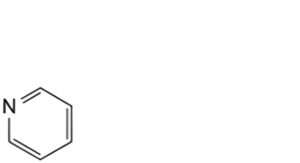
G. pyridine,
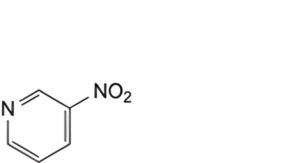
H. 3-nitropyridine,
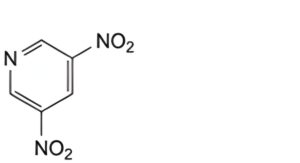
I. 3,5-dinitropyridine.