Action and use
Central nervous system stimulant; nicotine replacement therapy.
DEFINITION
Nicotine Sublingual Tablets contain Nicotine as a β-cyclodextrin complex.
The sublingual tablets comply with the requirements stated under Oromucosal Preparations and with the following requirements.
Content of nicotine C10H14N2
95.0 to 105.0% of the stated amount.
Carry out all of the following procedures protected from light.
IDENTIFICATION
Mix a quantity of the powdered tablets containing the equivalent of 20 mg of nicotine with 10 mL of chloroform. Disperse with the aid of ultrasound for 30 minutes and centrifuge for 10 minutes. Cool the mixture to 15°, add two 3-mL quantities of 0.5M hydrochloric acid and mix carefully. Centrifuge the mixture for 10 minutes. Transfer 5 mL of the aqueous layer to a separating funnel and add sufficient 0.5M sodium hydroxide to obtain a pH of 10.5, add 3 mL of chloroform, shake and retain the chloroform layer. The infrared absorption spectrum of the solution, Appendix II A, is concordant with the reference spectrum of nicotine (RS 452).
TESTS
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in 0.2M potassium dihydrogen orthophosphate adjusted to pH 2.0 with orthophosphoric acid (solvent A).
(1) To a quantity of the powdered tablets containing the equivalent of 20 mg of nicotine add 50 mL of solvent A, mix with the aid of ultrasound and filter through a 0.7-μm glass filter.
(2) Dilute 1 volume of solution (1) to 100 volumes.
(3) Dilute 1 volume of solution (2) to 10 volumes.
(4) 0.124% w/v of nicotine impurity standard BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped polar-embedded octadecylsilyl amorphous organosilica polymer (3.5 μm) (Waters XBridge is suitable) fitted with a guard column (3 cm × 4.6 mm) packed with the same material.
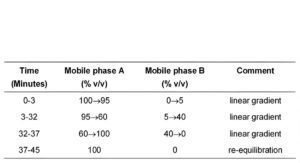
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 254 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
Mobile phase A: Dilute 25 volumes of 1M acetic acid to 1000 volumes with water, add 6.2 volumes of 18M ammonia and adjust the pH to 10 with 18M ammonia.
Mobile phase B: acetonitrile.
In the chromatogram obtained with solution (4):
identify the peaks due to cotinine, myosmine, cis-nicotine-1′-oxide and trans-nicotine-1′-oxide.
In the chromatogram obtained with solution (1):
identify any peak corresponding to cis-nicotine-1′-oxide and multiply the area of this peak by a correction factor of 1.5;
identify any peak corresponding to trans-nicotine-1′-oxide and multiply the area of this peak by a correction factor of 1.5.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between cotinine and trans- nicotine-1′-oxide is at least 2.0.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to cotinine is not greater than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the area of any peak corresponding to myosmine is not greater than 0.7 times the area of the principal peak in the chromatogram obtained with solution (2) (0.7%);
the area of any peak corresponding to cis-nicotine-1′-oxide is not greater than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the area of any peak corresponding to trans-nicotine-1′-oxide is not greater than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the area of any other secondary peak is not greater than 0.2 times the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the sum of the areas of any other secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.0%);
the sum of the areas of all the secondary peaks is not greater than five times the area of the principal peak in the chromatogram obtained with solution (2) (5.0%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (3) (0.1%).
Uniformity of content
Tablets containing less than 2 mg and/or less than 2% w/w of nicotine comply with the requirements stated under Oromucosal Preparations using the following method of analysis. Carry out the method for liquid chromatography, Appendix III D, using the following solutions in 0.2M potassium dihydrogen orthophosphate adjusted to pH 2.0 with orthophosphoric acid (solvent A).
(1) To one finely-powdered tablet add 50 mL of solvent A, mix with the aid of ultrasound and filter through a 0.7-μm glass filter.
(2) Prepare a suitable solution of nicotine ditartrate dihydrate BPCRS in solvent A.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
DETERMINATION OF CONTENT
Calculate the total content of C10H14N2 in each tablet using the declared content of C10H14N2 in nicotine ditartrate dihydrate BPCRS. Each mg of C10H14N2 is equivalent to 3.074 mg of C10H14N2,C8H12O12,2H2O.
ASSAY
For tablets containing less than 2 mg and/or less than 2% w/w of nicotine.
Use the average of the individual results determined in the test for Uniformity of content.
For tablets containing 2 mg or more and 2% w/w or more of nicotine.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in 0.2M potassium dihydrogen orthophosphate adjusted to pH 2.0 with orthophosphoric acid (solvent A).
(1) To a quantity of the powdered tablets containing the equivalent of 20 mg of nicotine add 50 mL of solvent A, mix with the aid of ultrasound and filter through a 0.7-μm glass filter. Dilute 2 mL of the resulting solution to 20 mL with solvent A.
(2) 0.0124% w/v of nicotine ditartrate dihydrate BPCRS.
(3) 0.0124% w/v of nicotine impurity standard BPCRS.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between cotinine and trans- nicotine-1′-oxide is at least 2.0.
DETERMINATION OF CONTENT
Calculate the total content of C10H14N2 in each tablet using the declared content of C10H14N2 in nicotine ditartrate dihydrate BPCRS. Each mg of C10H14N2 is equivalent to 3.074 mg of C10H14N2,C8H12O12,2H2O.
IMPURITIES
The impurities limited by the requirements of this monograph include those listed under Nicotine.






