Action and use
Central nervous system stimulant; nicotine replacement therapy.
DEFINITION
Nicotine Nasal Spray is a solution of Nicotine containing suitable buffering agents in a suitable container fitted with a suitable nasal delivery system.
The nasal spray complies with the requirements stated under Nasal Preparations and with the following requirements.
Content of nicotine, C10H14N2
95.0 to 105.0% of the stated amount.
Carry out all of the following procedures protected from light.
CHARACTERISTICS
Colourless or brownish solution.
IDENTIFICATION
To a volume of the nasal spray containing 20 mg of Nicotine add 5 mL of chloroform, dissolve with the aid of ultrasound and centrifuge for 10 minutes. Cool the mixture, add two 3-mL quantities of 0.5M hydrochloric acid and mix carefully. Centrifuge the mixture for 10 minutes. Transfer 5 mL of the aqueous layer to a separating funnel and add sufficient 0.5M sodium hydroxide to obtain a pH of 10.5, add 3 mL of chloroform, shake and retain the chloroform layer. The infrared absorption spectrum of the solution, Appendix II A, is concordant with the reference spectrum of nicotine (RS 452).
TESTS
Acidity or alkalinity
pH, 6.7 to 7.3, Appendix V L.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in 0.2M potassium dihydrogen orthophosphate adjusted to pH 2.0 with orthophosphoric acid (solvent A).
(1) Dilute a volume of the nasal spray containing 20 mg of Nicotine to 50 mL with solvent A and mix.
(2) Dilute 1 volume of solution (1) to 100 volumes.
(3) Dilute 1 volume of solution (2) to 10 volumes.
(4) 0.04% w/v of nicotine impurity standard BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped polar-embedded octadecylsilyl amorphous organosilica polymer (3.5 μm) (Waters XBridge is suitable) fitted with a guard column (3 cm × 4.6 mm) packed with the same material.
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 254 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
Mobile phase A: Add 25 volumes of 1M acetic acid to 1000 volumes of water, add 6.2 volumes of 18M ammonia and adjust the pH to 10 with 18M ammonia.
Mobile phase B: acetonitrile.
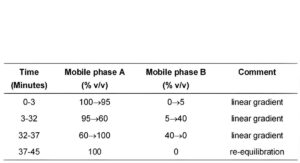
In the chromatogram obtained with solution (4):
identify the peaks due to cotinine, myosmine, cis-nicotine-1′-oxide and trans-nicotine-1′-oxide.
In the chromatogram obtained with solution (1):
Identify any peak corresponding to cis-nicotine-1′-oxide and multiply the area of this peak by a correction factor of 1.5;
identify any peak corresponding to trans-nicotine-1′-oxide and multiply the area of this peak by a correction factor of 1.5.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between trans-nicotine-1′-oxide and cotinine is at least 2.0.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to cotinine is not greater than 0.6 times the area of the principal peak in the chromatogram obtained with solution (2) (0.6%);
the area of any peak corresponding to myosmine is not greater than 0.7 times the area of the principal peak in the chromatogram obtained with solution (2) (0.7%);
the area of any peak corresponding to cis-nicotine-1′-oxide is not greater than 3 times the area of the principal peak in the chromatogram obtained with solution (2) (3.0%);
the area of any peak corresponding to trans-nicotine-1′-oxide is not greater than 3 times the area of the principal peak in the chromatogram obtained with solution (2) (3.0%);
the area of any other secondary peak is not greater than 0.2 times the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the sum of the areas of any other secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.0%);
the sum of the areas of all the secondary peaks is not greater than five times the area of the principal peak in the chromatogram obtained with solution (2) (5.0%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (3) (0.1%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in 0.2M potassium dihydrogen orthophosphate, the pH of which is adjusted to 2.0 with orthophosphoric acid (solvent A).
(1) To a volume of the nasal spray containing 20 mg of Nicotine add 50 mL of solvent A and mix. Dilute 1 volume of the resulting solution to 10 volumes.
(2) 0.0124% w/v of nicotine ditartrate dihydrate BPCRS in solvent A
(3) 0.04% w/v of nicotine impurity standard BPCRS in solvent A.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between trans-nicotine-1′-oxide and cotinine is at least 2.0.
DETERMINATION OF CONTENT
Calculate the total content of C10H14N2 in the nasal spray using the declared content of C10H14N2 in nicotine ditartrate dihydrate BPCRS. Each mg of C10H14N2 is equivalent to 3.074 mg of C10H14N2,C8H12O12,2H2O.
IMPURITIES
The impurities limited by the requirements of this monograph include those listed under Nicotine.






