Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Anthelminthic.
Preparation
Niclosamide Tablets Ph Eur
DEFINITION
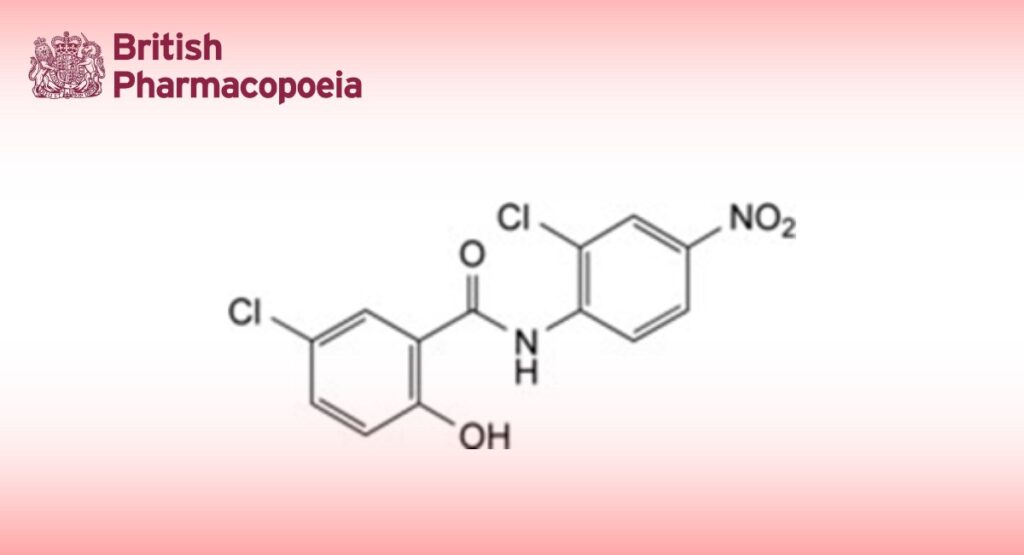
5-Chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide.
Content
98.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
Yellowish-white or yellowish, fine crystals.
Solubility
Practically insoluble in water, sparingly soluble in acetone, slightly soluble in anhydrous ethanol.
IDENTIFICATION
First identification: B, E.
Second identification: A, C, D, E.
A. Melting point (2.2.14): 227 °C to 232 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Preparation Discs prepared using about 0.5 mg of substance and 0.3 g of potassium bromide R. Comparison anhydrous niclosamide CRS.
C. To 50 mg add 5 mL of 1 M hydrochloric acid and 0.1 g of zinc powder R, heat in a water-bath for 10 min, cool and filter. To the filtrate add 1 mL of a 5 g/L solution of sodium nitrite R and allow to stand for 3 min; add 2 mL of a 20 g/L solution of ammonium sulfamate R, shake, allow to stand for 3 min and add 2 mL of a 5 g/L solution of naphthylethylenediamine dihydrochloride R. A violet colour is produced.
D. Heat the substance on a copper wire in a non-luminous flame. The flame becomes green.
E. Loss on drying (see Tests).
TESTS
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 50 mg of the substance to be examined in methanol R, heating gently, cool and dilute to 50.0 mL with the same solvent.
Reference solution Dilute 1.0 mL of the test solution to 100.0 mL with acetonitrile R. Dilute 1.0 mL of this solution to 20.0 mL with acetonitrile R.
Column:
— size: l = 0.125 m, Ø = 4 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase Mixture of equal volumes of acetonitrile R and a solution containing 2 g/L of potassium dihydrogen phosphate R, 1 g/L of disodium hydrogen phosphate dodecahydrate R and 2 g/L of tetrabutylammonium hydrogen sulfate R.
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 230 nm.
Injection 20 µL.
Run time Twice the retention time of niclosamide.
Limits:
— total: not more than 4 times the area of the principal peak in the chromatogram obtained with the reference solution (0.2 per cent);
— disregard limit: 0.1 times the area of the principal peak in the chromatogram obtained with the reference solution (0.005 per cent).
5-Chlorosalicylic acid
Maximum 60 ppm.
Test solution To 1.0 g add 15 mL of water R, boil for 2 min, cool, filter through a membrane filter (nominal pore size 0.45 µm), wash the filter and dilute the combined filtrate and washings to 20.0 mL with water R.
Reference solution Dissolve 30 mg of 5-chlorosalicylic acid R in 20 mL of methanol R and dilute to 100.0 mL with water R. Dilute 1.0 mL of this solution to 100.0 mL with water R.
To 10.0 mL of the test solution and to 10.0 mL of the reference solution add separately 0.1 mL of ferric chloride solution R2. Any violet colour in the test solution is not more intense than that in the reference solution.
2-Chloro-4-nitroaniline
Maximum 100 ppm.
Test solution To 0.250 g add 5 mL of methanol R, heat to boiling, cool, add 45 mL of 1 M hydrochloric acid, heat again to boiling, cool, filter and dilute the filtrate to 50.0 mL with 1 M hydrochloric acid.
Reference solution Dissolve 50 mg of 2-chloro-4-nitroaniline R in methanol R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with methanol R. Dilute 2.0 mL of this solution to 20.0 mL with 1 M hydrochloric acid.
To 10.0 mL of the test solution and to 10.0 mL of the reference solution add separately 0.5 mL of a 5 g/L solution of sodium nitrite R and allow to stand for 3 min. Add 1 mL of a 20 g/L solution of ammonium sulfamate R, shake, allow to stand for 3 min and add 1 mL of a 5 g/L solution of naphthylethylenediamine dihydrochloride R.
Any pinkish-violet colour in the test solution is not more intense than that in the reference solution.
Chlorides (2.4.4)
Maximum 500 ppm.
To 2 g add a mixture of 1.2 mL of acetic acid R and 40 mL of water R, boil for 2 min, cool and filter. Dilute 2 mL of the filtrate to 15 mL with water R.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 4 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.3000 g in 80 mL of a mixture of equal volumes of acetone R and methanol R. Titrate with 0.1 M tetrabutylammonium hydroxide, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M tetrabutylammonium hydroxide is equivalent to 32.71 mg of C13H8Cl2N2O4.
STORAGE
In an airtight container, protected from light. Ph Eur