Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Calcium channel blocker.
Ph Eur
DEFINITION
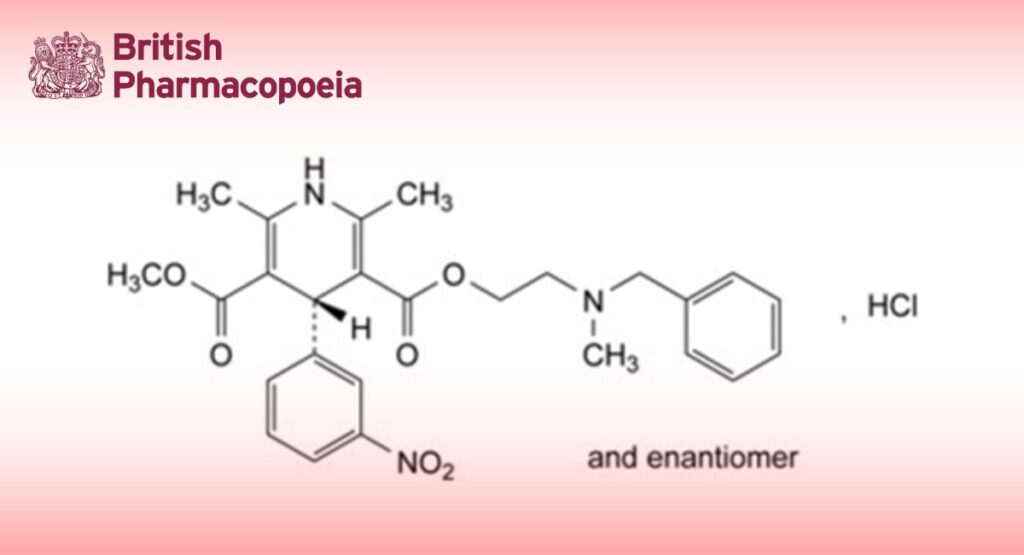
3-[2-[Benzyl(methyl)amino]ethyl] 5-methyl (4RS)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5- dicarboxylate hydrochloride.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
Pale yellow or pale greenish-yellow, crystalline powder.
Solubility
Slightly soluble in water, soluble in methanol, sparingly soluble in ethanol (96 per cent). It shows polymorphism (5.9).
IDENTIFICATION
First identification: A, C.
Second identification: B.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison nicardipine hydrochloride CRS.
If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in the minimum volume of methanol R, evaporate to dryness and record new spectra using the residues.
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 10 mg of the substance to be examined in methanol R and dilute to 10.0 mL with the same solvent.
Reference solution Dissolve 10 mg of nicardipine hydrochloride CRS in methanol R and dilute to 10.0 mL with the same solvent.
Plate TLC silica gel F254 plate R.
Mobile phase cyclohexane R, ethyl acetate R (40:60 V/V). Application 2 µL.
Development Over 3/4 of the plate.
Drying In air.
Detection A Examine in ultraviolet light at 254 nm.
Results A The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution.
Detection B Spray with iodoplatinate reagent R; examine the chromatogram in daylight.
Results B The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. Dissolve 30 mg in 10 mL of water R and shake vigorously. The solution gives reaction (a) of chlorides (2.3.1).
TESTS
Related substances
Liquid chromatography (2.2.29). Carry out the test protected from light.
Test solution Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 25.0 mL with the mobile phase.
Reference solution (a) Dilute 1.0 mL of the test solution to 50.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 20.0 mL with the mobile phase.
Reference solution (b) Dissolve 4 mg of nicardipine for system suitability CRS (containing impurities A, B and C) in 2 mL of the mobile phase.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 µm);
— temperature: 30 °C.
Mobile phase Mix 35 volumes of acetonitrile R and 65 volumes of a 1.5 g/L solution of perchloric acid R. Flow rate 1.5 mL/min.
Detection Spectrophotometer at 254 nm.
Injection 10 µL.
Run time 4 times the retention time of nicardipine.
Identification of impurities Use the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A, B and C.
Relative retention With reference to nicardipine (retention time = about 8 min): impurity B = about 0.5; impurity A = about 0.8; impurity C = about 2.1.
System suitability Reference solution (b):
— resolution: minimum 1.5 between the peaks due to impurity A and nicardipine.
Calculation of percentage contents:
— for each impurity, use the concentration of nicardipine hydrochloride in reference solution (a).
Limits:
— impurity B: maximum 0.5 per cent;
— impurities A, C: for each impurity, maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 1.0 per cent;
— reporting threshold: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 2 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.400 g in 50 mL of ethanol (96 per cent) R. Titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M sodium hydroxide is equivalent to 51.60 mg of C26H30ClN3O6.
STORAGE
In an airtight container, protected from light.
IMPURITIES
Specified impurities A, B, C.
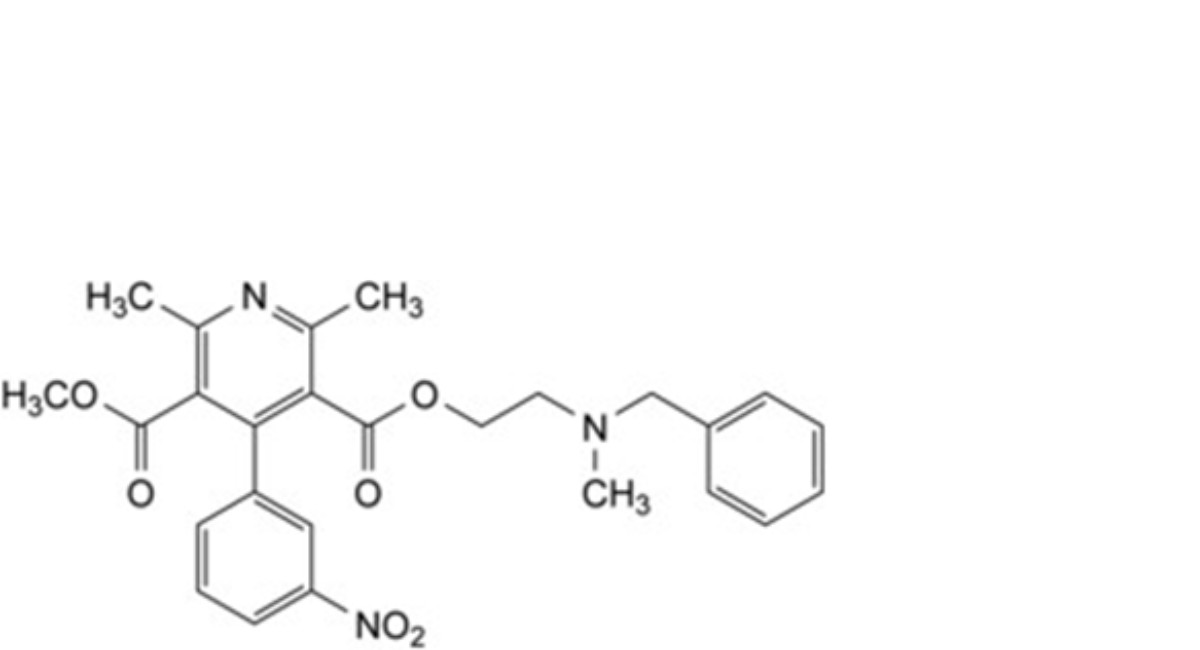
A. 3-[2-[benzyl(methyl)amino]ethyl] 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate,
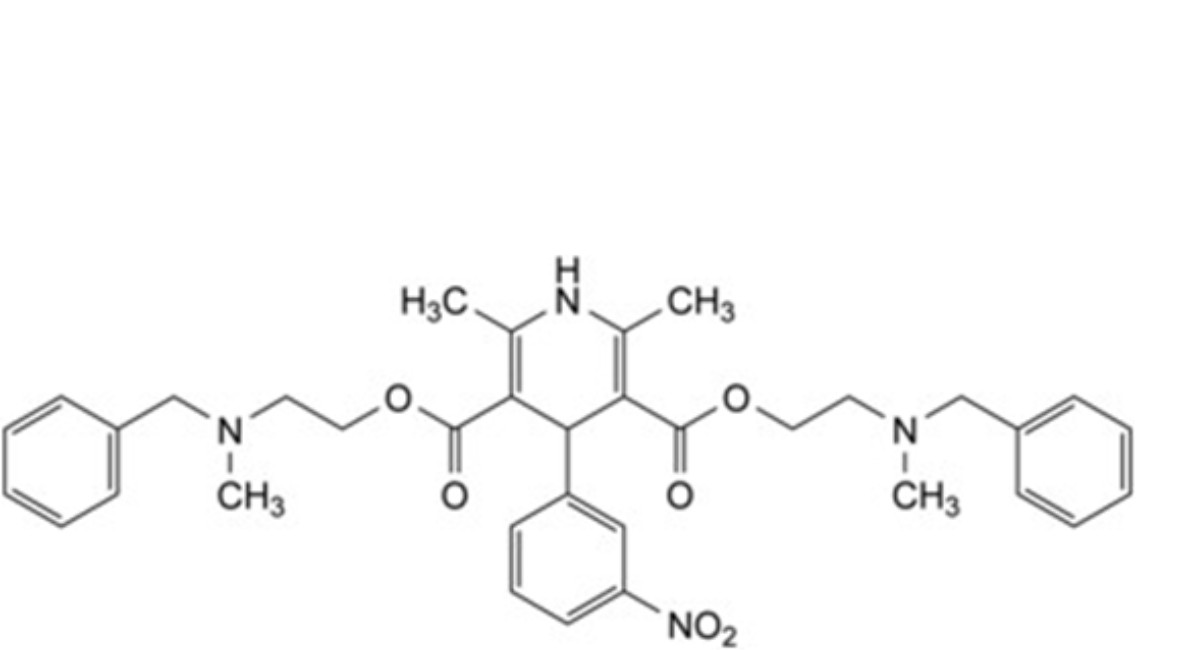
B. bis[2-[benzyl(methyl)amino]ethyl] 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate,
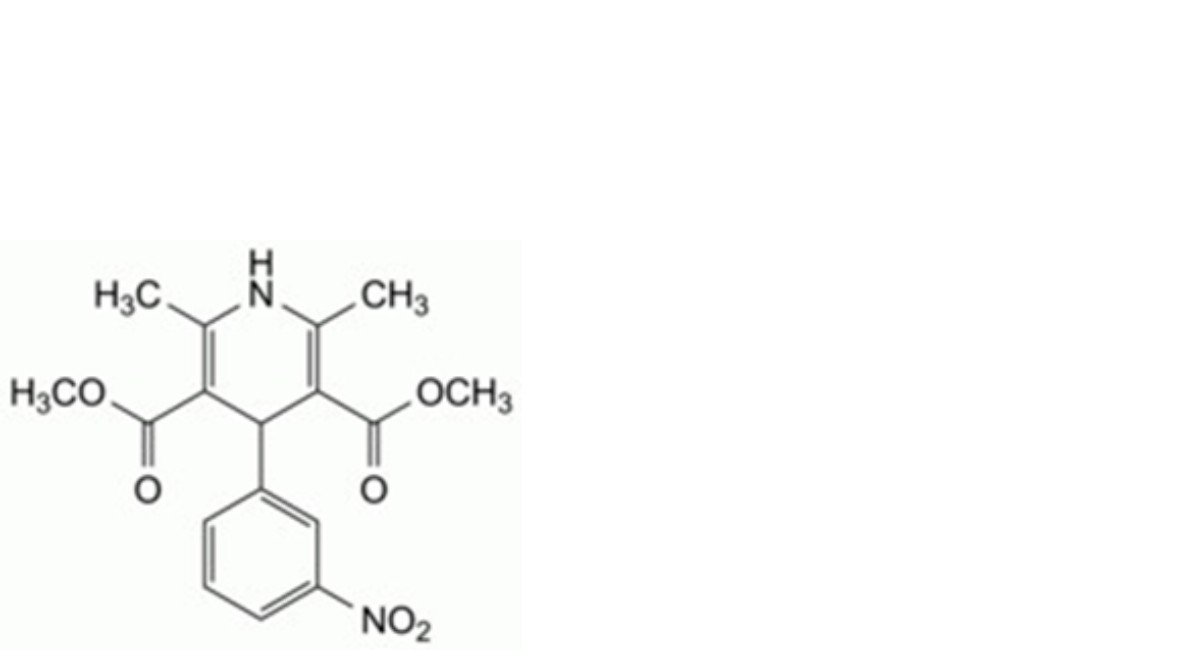
C. dimethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate.
Ph Eur