Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Non-nucleoside reverse transcriptase inhibitor; antiviral (HIV).
Preparations
Nevirapine Tablets
Nevirapine Prolonged-release Tablets
Ph Eur
DEFINITION
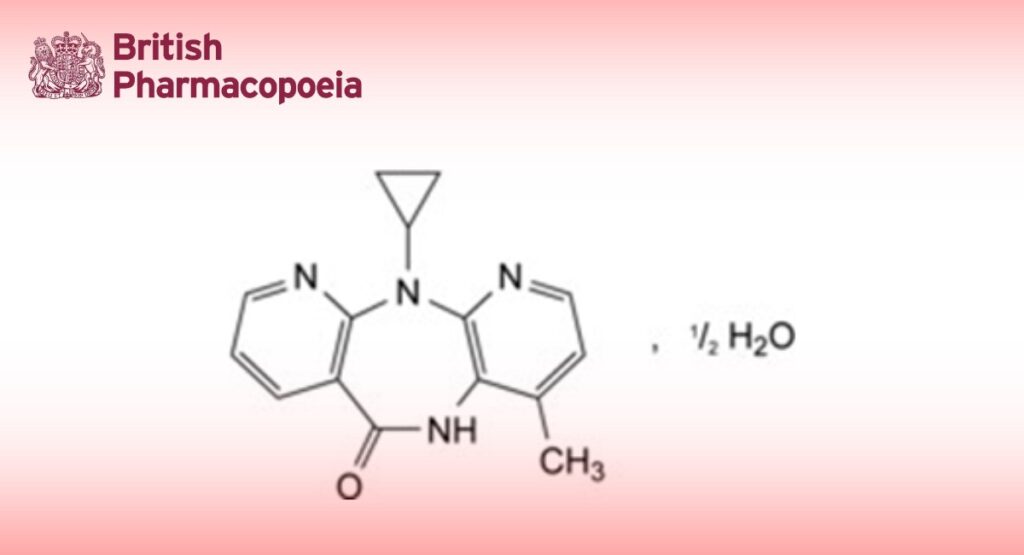
11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido [3,2-b:2′,3′-e][1,4]diazepin-6-one.
Content
97.5 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Practically insoluble in water, sparingly soluble or slightly soluble in methylene chloride, slightly soluble in methanol.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison anhydrous nevirapine CRS.
B. Loss on drying (see Tests).
TESTS
Related substances
Liquid chromatography (2.2.29).
Test solution (a) Dissolve 24.0 mg of the substance to be examined in a mixture of 4 mL of acetonitrile R and 80 mL of the mobile phase and sonicate until dissolution is complete. Dilute to 100.0 mL with the mobile phase.
Test solution (b) Dilute 3.0 mL of test solution (a) to 25.0 mL with the mobile phase.
Reference solution (a) Dilute 1.0 mL of test solution (a) to 100.0 mL with the mobile phase. Dilute 5.0 mL of this solution to 50.0 mL with the mobile phase.
Reference solution (b) Add 2.0 mL of the mobile phase to a vial of nevirapine for peak identification CRS
(containing impurities A, B and C), mix and sonicate for 1 min.
Reference solution (c) Dissolve 24.0 mg of anhydrous nevirapine CRS in a mixture of 4 mL of acetonitrile R and 80 mL of the mobile phase and sonicate until complete dissolution. Dilute to 100.0 mL with the mobile phase. Dilute 3.0 mL of this solution to 25.0 mL with the mobile phase.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped amidohexadecylsilyl silica gel for chromatography R (5 µm);
— temperature: 35 °C.
Mobile phase Mix 20 volumes of acetonitrile R and 80 volumes of a 2.88 g/L solution of ammonium dihydrogen phosphate R, previously adjusted to pH 5.0 using dilute sodium hydroxide solution R.
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 220 nm.
Injection 50 µL of test solution (a) and reference solutions (a) and (b).
Run time 10 times the retention time of nevirapine.
Identification of impurities Use the chromatogram supplied with nevirapine for peak identification CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A, B and C.
Relative retention With reference to nevirapine (retention time = about 8 min): impurity B = 0.7; impurity A = 1.5; impurity C = 2.8.
System suitability Reference solution (b):
— resolution: minimum 5 between the peaks due to impurity B and nevirapine.
Limits:
— impurities A, B, C: for each impurity, not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent);
— total: not more than 6 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.6 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection 25 µL of test solution (b) and reference solution (c).
Calculate the percentage content of C15H14N4O from the declared content of anhydrous nevirapine CRS.
IMPURITIES
Specified impurities A, B, C.
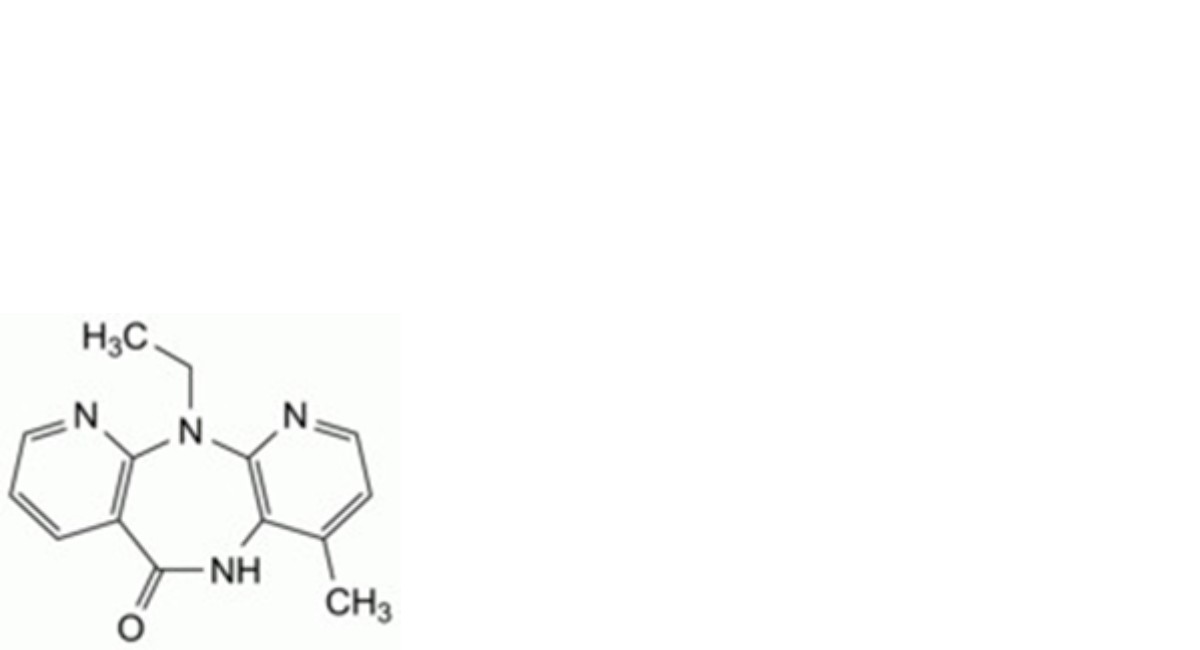
A. 11-ethyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2′,3′-e][1,4]diazepin-6-one,
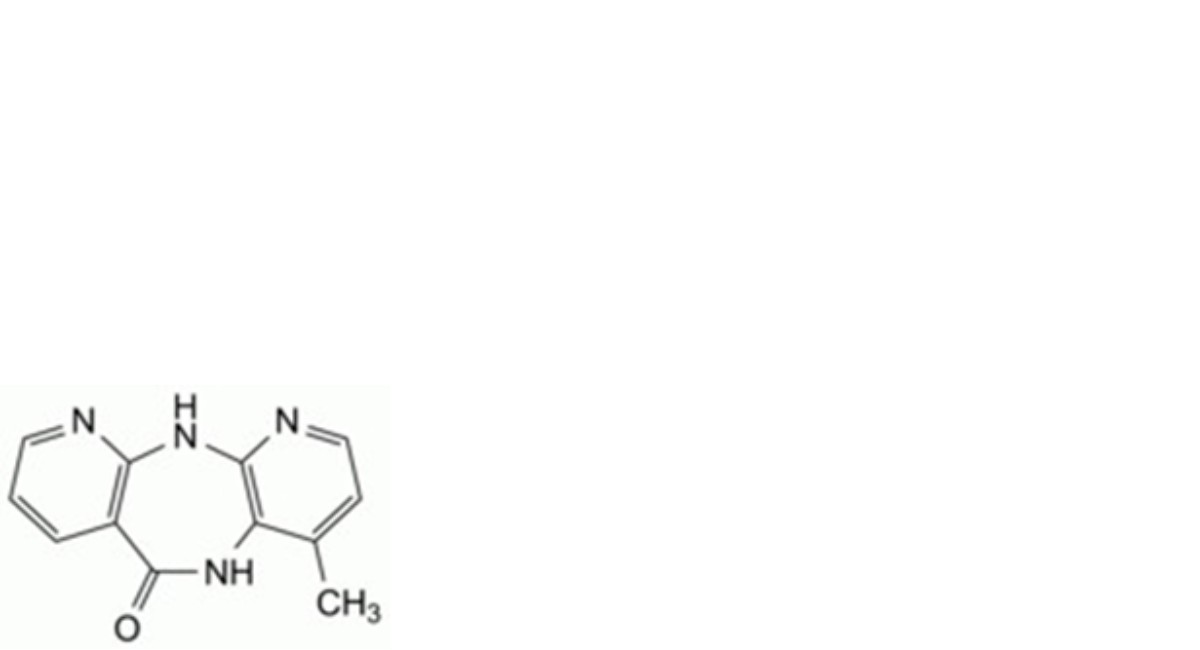
B. 4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2′,3′-e][1,4]diazepin-6-one,
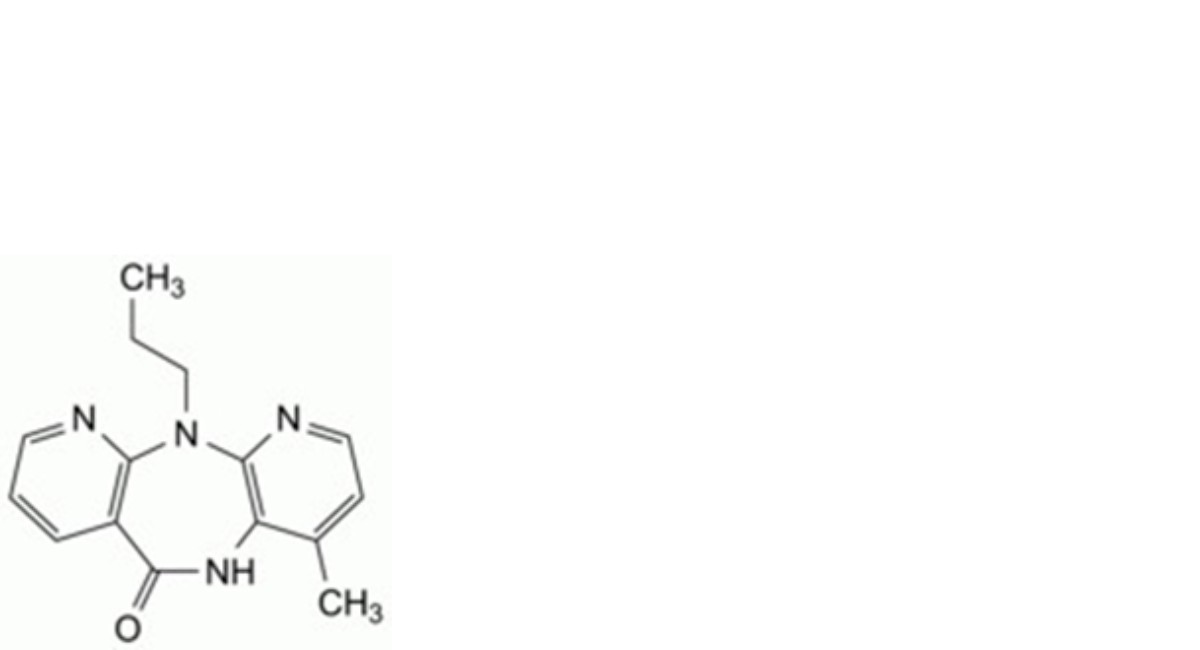
C. 4-methyl-11-propyl-5,11-dihydro-6H-dipyrido[3,2-b:2′,3′-e][1,4]diazepin-6-one.
Ph Eur