Edition: BP 2025 (Ph. Eur. 11.6 update)
DEFINITION
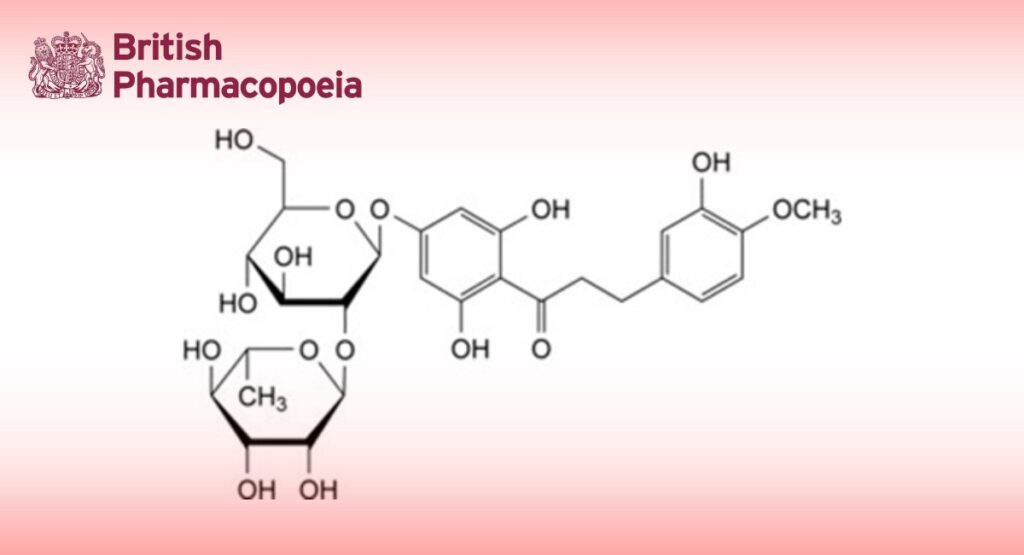
1-[4-[[2-O-(6-Deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-2,6-dihydroxyphenyl]-3-(3-hydroxy-4- methoxyphenyl)propan-1-one.
Content
96.0 per cent to 101.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or yellowish-white powder.
Solubility
Practically insoluble in water, freely soluble in dimethyl sulfoxide, soluble in methanol, practically insoluble in methylene chloride.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison neohesperidin-dihydrochalcone CRS.
B. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with test solution (b) is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution Y4 (2.2.2, Method II). Dissolve 0.25 g in methanol R and dilute to 25 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution (a) Dissolve 0.10 g of the substance to be examined in dimethyl sulfoxide R and dilute to 50.0 mL with the same solvent.
Test solution (b) Dilute 10.0 mL of test solution (a) to 20.0 mL with dimethyl sulfoxide R.
Reference solution (a) Dissolve 50.0 mg of neohesperidin-dihydrochalcone CRS in dimethyl sulfoxide R and dilute to 50.0 mL with the same solvent.
Reference solution (b) Dissolve 4.0 mg of neohesperidin-dihydrochalcone impurity B CRS in dimethyl sulfoxide R and dilute to 100.0 mL with the same solvent.
Reference solution (c) Dilute 1.0 mL of test solution (a) to 100.0 mL with dimethyl sulfoxide R.
Reference solution (d) In order to prepare in situ impurity F and impurity G, suspend 0.10 g of the substance to be examined in 10.0 mL of a 100 g/L solution of sulfuric acid R. Heat the sample for 5 min on a water-bath. Dilute immediately 1.0 mL of the resulting solution to 50.0 mL with dimethyl sulfoxide R.
Column:
— size: l = 0.15 m, Ø = 3.9 mm,
— stationary phase: spherical octadecylsilyl silica gel for chromatography R (4 µm) with a carbon loading of 7 per cent,
— temperature: 30 °C.
Mobile phase Mix 20 volumes of acetonitrile R and 80 volumes of a solution prepared by adding 5.0 mL of glacial acetic acid R to 1000.0 mL of water R. Flow rate 1.0 mL/min.
Detection Spectrophotometer at 282 nm.
Injection 10 µL; inject test solution (a) and reference solutions (a), (b), (c) and (d).
Run time 5 times the retention time of neohesperidin-dihydrochalcone which is about 10 min.
Relative retention With reference to neohesperidin-dihydrochalcone: impurity B = about 0.4; impurity D = about 0.7; impurity F = about 1.2; impurity G = about 3.7.
System suitability:
— resolution: minimum of 2.5 between the first peak (neohesperidin-dihydrochalcone) and the second peak (impurity F) in the chromatogram obtained with reference solution (d),
— chromatogram obtained with reference solution (a) is similar to the chromatogram provided with
neohesperidin-dihydrochalcone CRS. Limits:
— impurity B: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (2 per cent),
— impurity D: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (c) (2 per cent),
— any other impurity: not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (c) (0.5 per cent),
— total of all impurities apart from impurity B: not more than 2.5 times the area of the principal peak in the chromatogram obtained with reference solution (c) (2.5 per cent),
— disregard limit: 0.05 times the area of the principal peak in the chromatogram obtained with reference solution (c) (0.05 per cent).
Water (2.5.12)
Maximum 12.0 per cent, determined on 0.200 g.
Sulfated ash (2.4.14)
Maximum 0.2 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances.
Injection 10 µL; inject test solution (b) and reference solutions (a) and (d).
System suitability:
— resolution: minimum of 2.5 between the first peak (neohesperidin-dihydrochalcone) and the second peak (impurity F) in the chromatogram obtained with reference solution (d),
— repeatability: reference solution (a).
Calculate the percentage content of C28H36O15 using the chromatogram obtained with reference solution (a) and the stated content of C28H36O15 in neohesperidin-dihydrochalcone CRS, correcting for the water content of the substance to be examined.
STORAGE
Protected from light.
IMPURITIES
A. 1-[4-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-2,6-dihydroxyphenyl]ethanone (phloroacetophenone neohesperidoside),
B. 7-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4- methoxyphenyl)-4H-1-benzopyran-4-one (neodiosmin),
C. (2RS)-7-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4- methoxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one (neohesperidin),
D. 1-[4-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-2,6-dihydroxyphenyl]-3-(4- hydroxyphenyl)propan-1-one (naringin-dihydrochalcone),
E. 1-[4-[[6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-2,6-dihydroxyphenyl]-3-(3-hydroxy-4- methoxyphenyl)propan-1-one (hesperidin-dihydrochalcone),
F. 1-[4-(β-D-glucopyranosyloxy)-2,6-dihydroxyphenyl]-3-(3-hydroxy-4-methoxyphenyl)propan-1-one (hesperetin-dihydrochalcone 7′-glucoside),
G. 3-(3-hydroxy-4-methoxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one (hesperetin-dihydrochalcone).