Edition: BP 2025 (Ph. Eur. 11.6 update)
DEFINITION
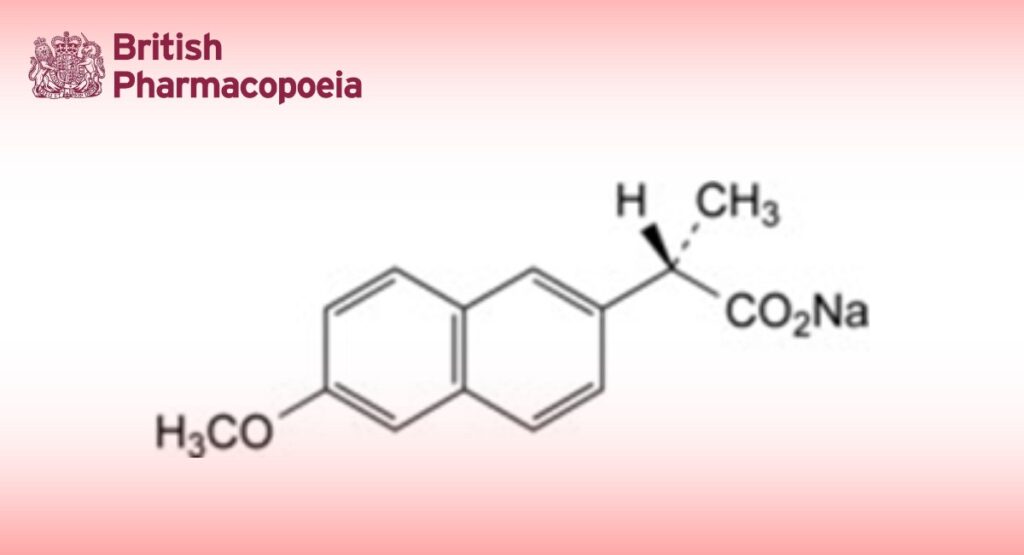
Sodium (2S)-2-(6-methoxynaphthalen-2-yl)propanoate.
Content
98.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, hygroscopic, crystalline powder.
Solubility
Freely soluble in water, freely soluble or soluble in methanol, sparingly soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: A, C, D.
Second identification: A, B, D.
A. Specific optical rotation (2.2.7): -17.0 to -14.7 (dried substance).
Dissolve 0.50 g in a 4.2 g/L solution of sodium hydroxide R and dilute to 25.0 mL with the same solution.
B. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution Dissolve 40.0 mg in methanol R and dilute to 100.0 mL with the same solvent. Dilute 10.0 mL of this solution to 100.0 mL with methanol R.
Spectral range 230-350 nm.
Absorption maxima At 262 nm, 271 nm, 316 nm and 331 nm.
Specific absorbance at the absorption maxima:
— at 262 nm: 207 to 227;
— at 271 nm: 200 to 220;
— at 316 nm: 56 to 68;
— at 331 nm: 72 to 84.
C. Infrared absorption spectrophotometry (2.2.24).
Preparation Dissolve 50 mg in 5 mL of water R. Add 1 mL of dilute sulfuric acid R and 5 mL of ethyl acetate R. Shake vigorously. Allow the 2 layers to separate.
Evaporate the upper layer to dryness and subsequently dry at 60 °C for 15 min. Record the spectrum using the residue.
Comparison naproxen CRS.
D. It gives reaction (a) of sodium (2.3.1).
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution BY7 (2.2.2, Method II). Dissolve 1.25 g in water R and dilute to 25 mL with the same solvent.
pH (2.2.3)
7.0 to 9.8.
Dissolve 0.5 g in carbon dioxide-free water R and dilute to 25 mL with the same solvent.
Enantiomeric purity
Liquid chromatography (2.2.29). Protect the solutions from light.
Test solution Dissolve 25.0 mg of the substance to be examined in 15 mL of water R and add 1 mL of hydrochloric acid R. Shake with 2 quantities, each of 10 mL, of ethyl acetate R, combine the upper layers and evaporate to dryness under reduced pressure. Dissolve the residue in 50.0 mL of tetrahydrofuran R. Dilute 2.0 mL of this solution to 20.0 mL with the mobile phase.
Reference solution (a) Dilute 2.5 mL of the test solution to 100.0 mL with the mobile phase.
Reference solution (b) Dissolve 5 mg of racemic naproxen CRS in 10 mL of tetrahydrofuran R and dilute to 100 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: silica gel π-acceptor/π-donor for chiral separations R (5 µm) (S,S);
— temperature: 25 °C.
Mobile phase glacial acetic acid R, acetonitrile R, 2-propanol R, hexane R (5:50:100:845 V/V/V/V). Flow rate 2 mL/min.
Detection Spectrophotometer at 263 nm.
Injection 20 µL.
Run time 1.5 times the retention time of naproxen (retention time = about 5 min).
System suitability Reference solution (b):
— resolution: minimum 3 between the peaks due to impurity G and naproxen.
Limit:
— impurity G: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (2.5 per cent).
Related substances
Liquid chromatography (2.2.29). Protect the solutions from light.
Test solution Dissolve 12 mg of the substance to be examined in the mobile phase and dilute to 20 mL with the mobile phase.
Reference solution (a) Dilute 1.0 mL of the test solution to 50.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 20.0 mL with the mobile phase.
Reference solution (b) Dissolve 6 mg of bromomethoxynaphthalene R (impurity N), 6.0 mg of naproxen impurity L CRS and 6 mg of (1RS)-1-(6-methoxynaphthalen-2-yl)ethanol R (impurity K) in acetonitrile R and dilute to 10 mL with the same solvent. To 1 mL of the solution add 1 mL of the test solution and dilute to 50 mL with the mobile phase. Dilute 1 mL of this solution to 20 mL with the mobile phase.
Column:
— size: l = 0.10 m, Ø = 4.0 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (3 µm);
— temperature: 50 °C.
Mobile phase Mix 42 volumes of acetonitrile R and 58 volumes of a 1.36 g/L solution of potassium dihydrogen phosphate R previously adjusted to pH 2.0 with phosphoric acid R.
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 230 nm.
Injection 20 µL.
Run time 1.5 times the retention time of impurity N.
Relative retention With reference to naproxen (retention time = about 2.5 min): impurity K = about 0.9; impurity L = about 1.4; impurity N = about 5.3.
System suitability Reference solution (b):
— resolution: minimum 2.2 between the peaks due to impurity K and naproxen.
Limits:
— impurity L: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.1 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
— total: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.3 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 3 h.
ASSAY
Dissolve 0.200 g in 50 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 25.22 mg of C14H13O3Na.
STORAGE
In an airtight container, protected from light.
IMPURITIES
Specified impurities G, L.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.
Control of impurities in substances for pharmaceutical use) A, B, C, D, E, F, H, I, J, K, M, N.
A. (2S)-2-(6-hydroxynaphthalen-2-yl)propanoic acid,
B. (2S)-2-(5-chloro-6-methoxynaphthalen-2-yl)propanoic acid,
C. (2S)-2-(5-bromo-6-methoxynaphthalen-2-yl)propanoic acid,
D. (2S)-2-(5-iodo-6-methoxynaphthalen-2-yl)propanoic acid,
E. methyl (2S)-2-(6-methoxynaphthalen-2-yl)propanoate,
F. ethyl (2S)-2-(6-methoxynaphthalen-2-yl)propanoate,
G. (2R)-2-(6-methoxynaphthalen-2-yl)propanoic acid,
H. 6-methoxynaphthalen-2-ol,
I. (6-methoxynaphthalen-2-yl)acetic acid,
J. 2-ethyl-6-methoxynaphthalene,
K. (1RS)-1-(6-methoxynaphthalen-2-yl)ethanol,
L. 1-(6-methoxynaphthalen-2-yl)ethanone,
M. 2-methoxynaphthalene (nerolin),
N. 2-bromo-6-methoxynaphthalene.