Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Alpha-adrenoceptor agonist. Ph Eur
DEFINITION
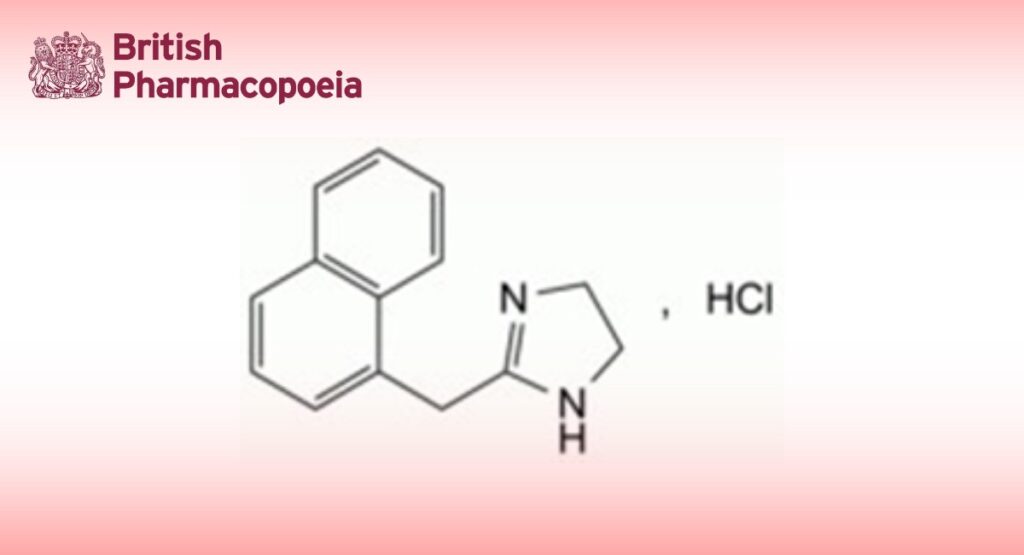
2-(Naphthalen-1-ylmethyl)-4,5-dihydro-1H-imidazole hydrochloride.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water, soluble in ethanol (96 per cent).
mp
About 259 °C, with decomposition.
IDENTIFICATION
First identification: B.
Second identification: A, C.
A. Dissolve 50.0 mg in 0.01 M hydrochloric acid and dilute to 250.0 mL with the same acid. Dilute 25.0 mL of the solution to 100.0 mL with 0.01 M hydrochloric acid. Examined between 230 nm and 350 nm (2.2.25), the solution shows 4 absorption maxima, at 270 nm, 280 nm, 287 nm and 291 nm. The ratios of the absorbances measured at the maxima at 270 nm, 287 nm and 291 nm to that measured at the maximum at 280 nm are 0.82 to 0.86, 0.67 to 0.70 and 0.65 to 0.69, respectively.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison naphazoline hydrochloride CRS.
C. It gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dissolve 0.5 g in carbon dioxide-free water R and dilute to 50 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
To 20 mL of solution S add 0.2 mL of 0.01 M sodium hydroxide and 0.1 mL of methyl red solution R. The solution is yellow. Not more than 0.6 mL of 0.01 M hydrochloric acid is required to change the colour of the solution to red.
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (a) Dissolve 5 mg of 1-naphthylacetic acid R in the mobile phase, add 5 mL of the test solution and dilute to 100 mL with the mobile phase.
Reference solution (b) Dissolve 5.0 mg of naphazoline impurity A CRS in the mobile phase and dilute to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 100.0 mL with the mobile phase.
Reference solution (c) Dilute 1.0 mL of the test solution to 10.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 100.0 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.0 mm;
— stationary phase: base-deactivated end-capped octylsilyl silica gel for chromatography R (4 µm) with a pore size of 6 nm.
Mobile phase Dissolve 1.1 g of sodium octanesulfonate R in a mixture of 5 mL of glacial acetic acid R, 300 mL of acetonitrile R and 700 mL of water R.
Flow rate 1 mL/min.
Detection Spectrophotometer at 280 nm.
Injection 20 µL.
Run time 3 times the retention time of naphazoline.
Retention time Naphazoline = about 14 min.
System suitability Reference solution (a):
— resolution: minimum 5.0 between the peaks due to naphazoline and impurity B.
Limits:
— impurity A: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.1 per cent);
— unspecified impurities: not more than the area of the principal peak in the chromatogram obtained with reference solution (c) (0.10 per cent);
— total: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (c) (0.5 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (c) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.200 g in a mixture of 5.0 mL of 0.01 M hydrochloric acid and 50 mL of ethanol (96 per cent) R. Carry out a potentiometric titration (2.2.20), using 0.1 M sodium hydroxide. Read the volume added between the 2 points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 24.67 mg of C14H15ClN2.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A.
Other detectable impurities: B, C, D.
A. N-(2-aminoethyl)-2-(naphthalen-1-yl)acetamide (naphthylacetylethylenediamine),
B. (naphthalen-1-yl)acetic acid (1-naphthylacetic acid),
C. (naphthalen-1-yl)acetonitrile (1-naphthylacetonitrile),
D. 2-(naphthalen-2-ylmethyl)-4,5-dihydro-1H-imidazole (β-naphazoline).
Ph Eur