Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Vasodilator.
Preparation
Naftidrofuryl Capsules
Ph Eur
DEFINITION
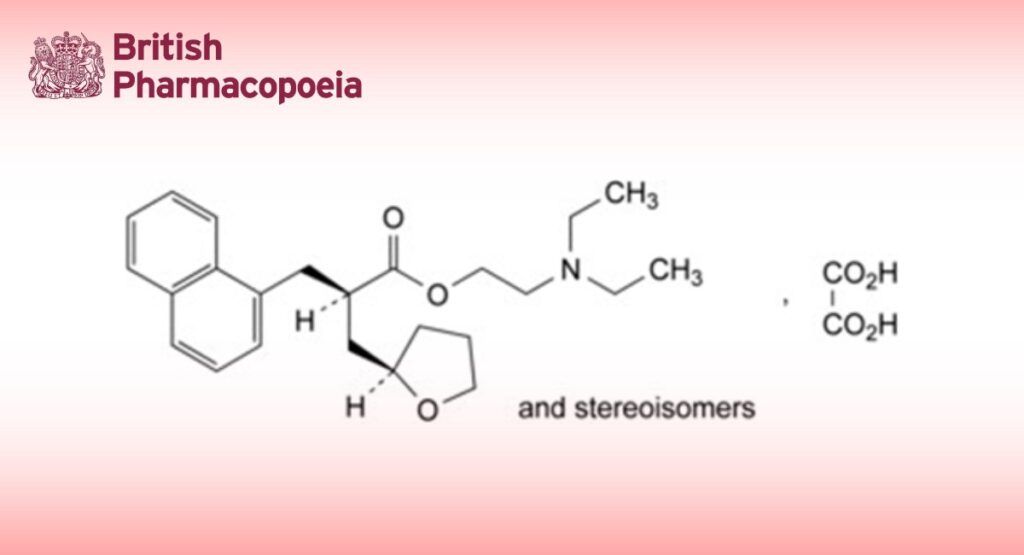
Mixture of 4 stereoisomers of 2-(diethylamino)ethyl 2-[(naphthalen-1-yl)methyl]-3-(tetrahydrofuran-2- yl)propanoate hydrogen oxalate.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Freely soluble in water, freely soluble or soluble in ethanol (96 per cent), slightly or sparingly soluble in acetone.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Preparation Dissolve 1.0 g in water R and dilute to 50 mL with the same solvent. Add 2 mL of concentrated ammonia R and shake with 3 quantities, each of 10 mL, of methylene chloride R. To the combined lower layers, add anhydrous sodium sulfate R, shake, filter and evaporate the filtrate by suitable means at a temperature not exceeding 30 °C. Use the residue obtained.
Comparison Ph. Eur. reference spectrum of naftidrofuryl.
B. Dissolve 0.5 g in water R and dilute to 10 mL with the same solvent. Add 2.0 mL of calcium chloride solution R. A white precipitate is formed. The precipitate dissolves after the addition of 3.0 mL of hydrochloric acid R.
TESTS
Absorbance (2.2.25)
Maximum 0.1 at 430 nm.
Dissolve 1.5 g in water R and dilute to 10 mL with the same solvent. If necessary use an ultrasonic bath.
Related substances
A. Liquid chromatography (2.2.29).
Test solution Dissolve 80.0 mg of the substance to be examined in the mobile phase and dilute to 20.0 mL with the mobile phase. Sonicate for 10 s. A precipitate is formed. Filter through a membrane filter (nominal pore size 0.45 µm), discarding the first 5 mL. Use a freshly prepared solution.
Reference solution (a) Dissolve 5.0 mg of naftidrofuryl impurity A CRS in acetonitrile R and dilute to 25.0 mL with the same solvent. Dilute 1.0 mL of the solution to 50.0 mL with the mobile phase.
Reference solution (b) Dissolve 5 mg of naftidrofuryl impurity B CRS and 5 mg of the substance to be examined in acetonitrile R and dilute to 50 mL with the same solvent. Dilute 1 mL of the solution to 50 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase Mix 60 mL of methanol R with 150 mL of tetrabutylammonium buffer solution pH 7.0 R and dilute to 1000 mL with acetonitrile R.
Flow rate 1 mL/min.
Detection Spectrophotometer at 283 nm.
Injection 20 µL.
Run time 2.3 times the retention time of naftidrofuryl.
Relative retention With reference to naftidrofuryl (retention time = about 7 min): impurity A = about 0.5; impurity B = about 0.8; impurity C = about 1.8.
System suitability Reference solution (b):
— resolution: minimum 3.0 between the peaks due to impurity B and naftidrofuryl.
Limits:
— impurities A, B, C: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent);
— any other impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent);
— total: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.3 per cent);
— disregard limit: 0.2 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.02 per cent).
B. Gas chromatography (2.2.28).
Test solution (a) Dissolve 1.0 g of the substance to be examined in water R and dilute to 50 mL with the same solvent. Add 2 mL of concentrated ammonia R and shake with 3 quantities, each of 10 mL, of methylene chloride R. To the combined lower layers, add anhydrous sodium sulfate R, shake, filter and evaporate the filtrate by suitable means at a temperature not exceeding 30 °C. Take up the residue with methylene chloride R and dilute to 20.0 mL with the same solvent.
Test solution (b) Dilute 1.0 mL of test solution (a) to 10.0 mL with methylene chloride R.
Reference solution Dissolve 5 mg of naftidrofuryl impurity F CRS in methylene chloride R and dilute to 50 mL with the same solvent.
Column:
— material: fused silica;
— size: l = 25 m, Ø = 0.32 mm;
— stationary phase: phenyl(5)methyl(95)polysiloxane R (film thickness 0.45 µm).
Carrier gas helium for chromatography R. Splitter flow rate 25 mL/min.
Flow rate 2.9 mL/min.
Temperature:
| Time (min) | Temperature (°C) | |
| Column | 0 – 4 | 210 |
| 4 – 8 | 210 → 230 | |
| 8 – 18 | 230 → 260 | |
| 18 – 30 | 260 | |
| Injection port | 290 | |
| Detector | 290 | |
Detection Flame ionisation.
Injection 1 µL.
Relative retention With reference to the second eluting peak of naftidrofuryl: impurity D = about 0.14; impurity B = about 0.55 (for the second eluting peak); impurity E = about 0.86; impurity F = about 1.04 (for the second eluting peak).
System suitability Test solution (b):
— resolution: minimum 1.0 between the 2 peaks due to the diastereoisomers of naftidrofuryl.
Limits Test solution (a):
— impurity F: for the sum of the areas of the 2 peaks, maximum 0.20 per cent of the sum of the areas of the 2 peaks due to naftidrofuryl (0.20 per cent);
— impurity E: maximum 0.20 per cent of the sum of the areas of the 2 peaks due to naftidrofuryl (0.20 per cent);
— impurity D: maximum 0.10 per cent of the sum of the areas of the 2 peaks due to naftidrofuryl (0.10 per cent);
— any other impurity: for each impurity, maximum 0.10 per cent of the sum of the areas of the 2 peaks due to naftidrofuryl (0.10 per cent);
— total: maximum 0.50 per cent of the sum of the areas of the 2 peaks due to naftidroduryl (0.50 per cent);
— disregard limit: 0.02 per cent of the sum of the areas of the 2 peaks due to naftidrofuryl (0.02 per cent); disregard any peaks due to impurity B.
Diastereoisomer ratio
Gas chromatography (2.2.28) as described in test B for related substances.
Limits Test solution (b):
— first eluting naftidrofuryl diastereoisomer: minimum 30 per cent of the sum of the areas of the 2 peaks due to naftidrofuryl.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.350 g in 50 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 47.36 mg of C26H35NO7.
IMPURITIES
Specified impurities A, B, C, D, E, F.
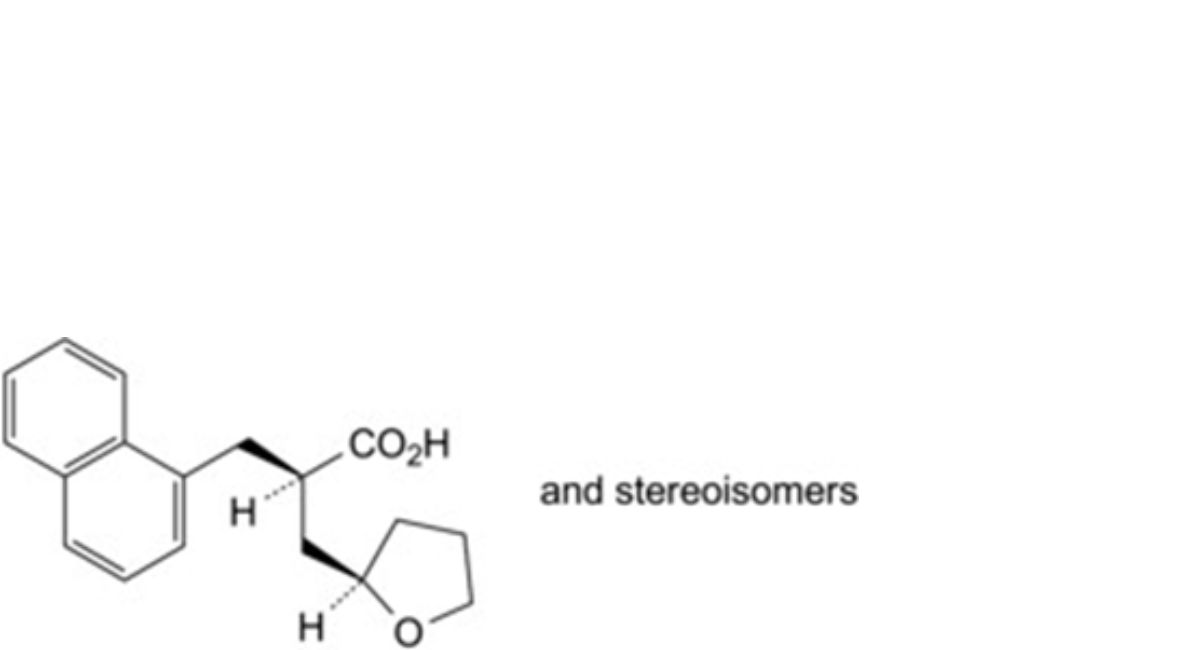
A. 2-[(naphthalen-1-yl)methyl]-3-(tetrahydrofuran-2-yl)propanoic acid,
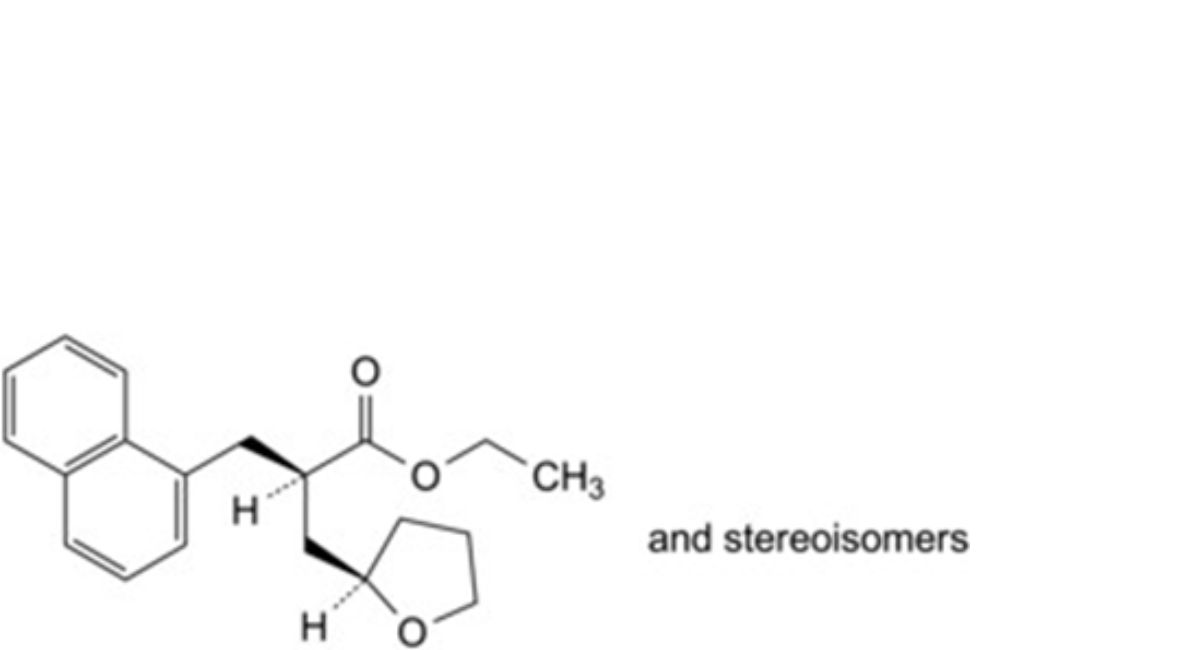
B. ethyl 2-[(naphthalen-1-yl)methyl]-3-(tetrahydrofuran-2-yl)propanoate,
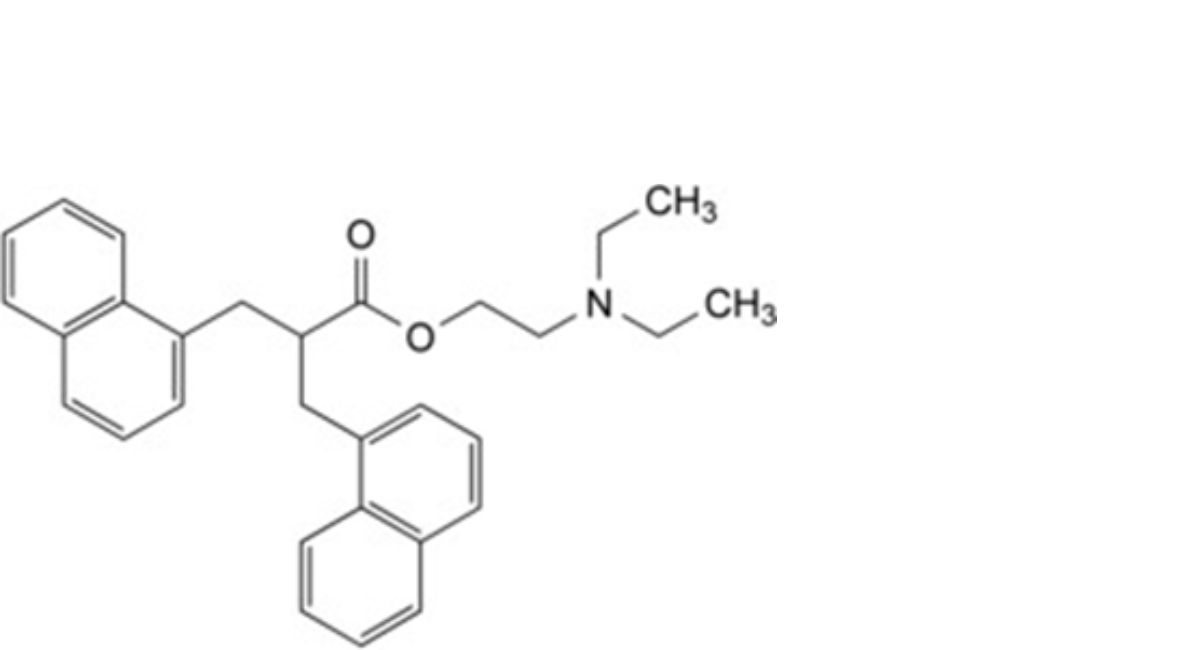
C. 2-(diethylamino)ethyl 3-(naphthalen-1-yl)-2-[(naphthalen-1-yl)methyl]propanoate,
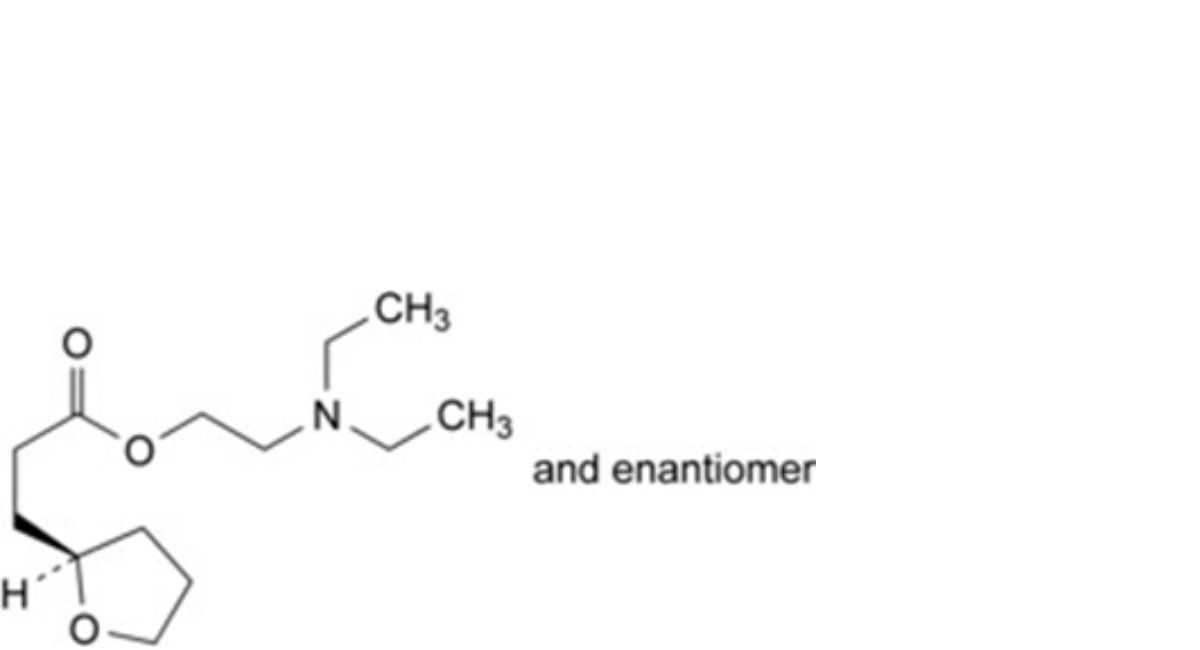
D. 2-(diethylamino)ethyl 3-[(2RS)-tetrahydrofuran-2-yl]propanoate,
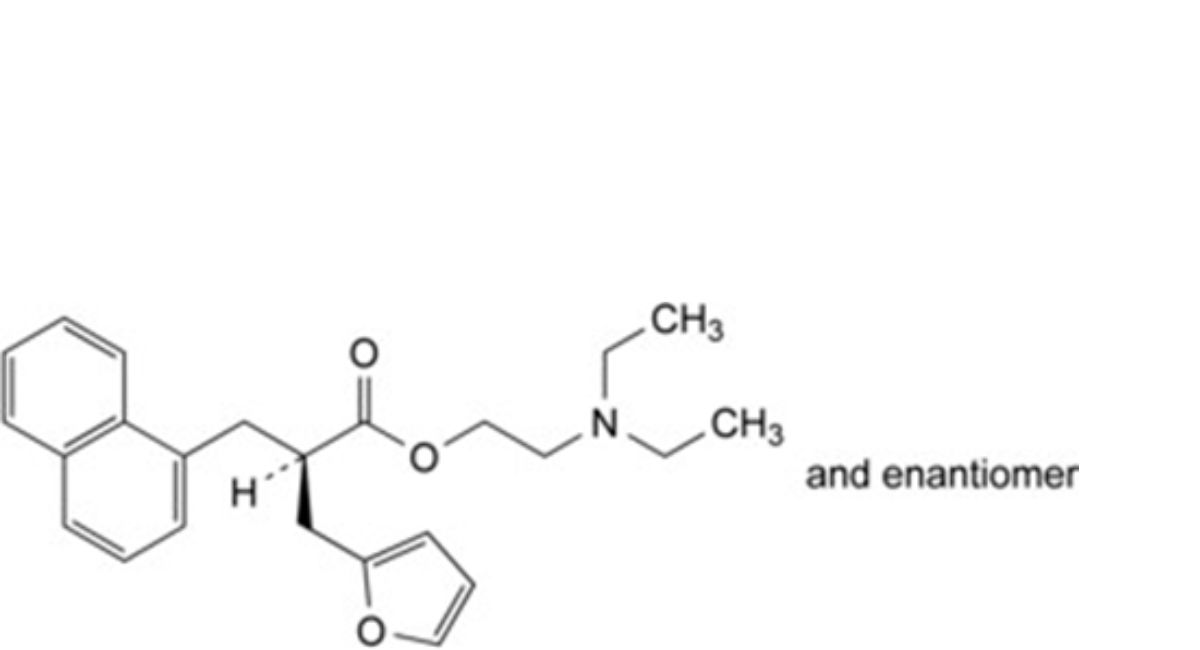
E. 2-(diethylamino)ethyl (2RS)-2-[(furan-2-yl)methyl]-3-(naphthalen-1-yl)propanoate,
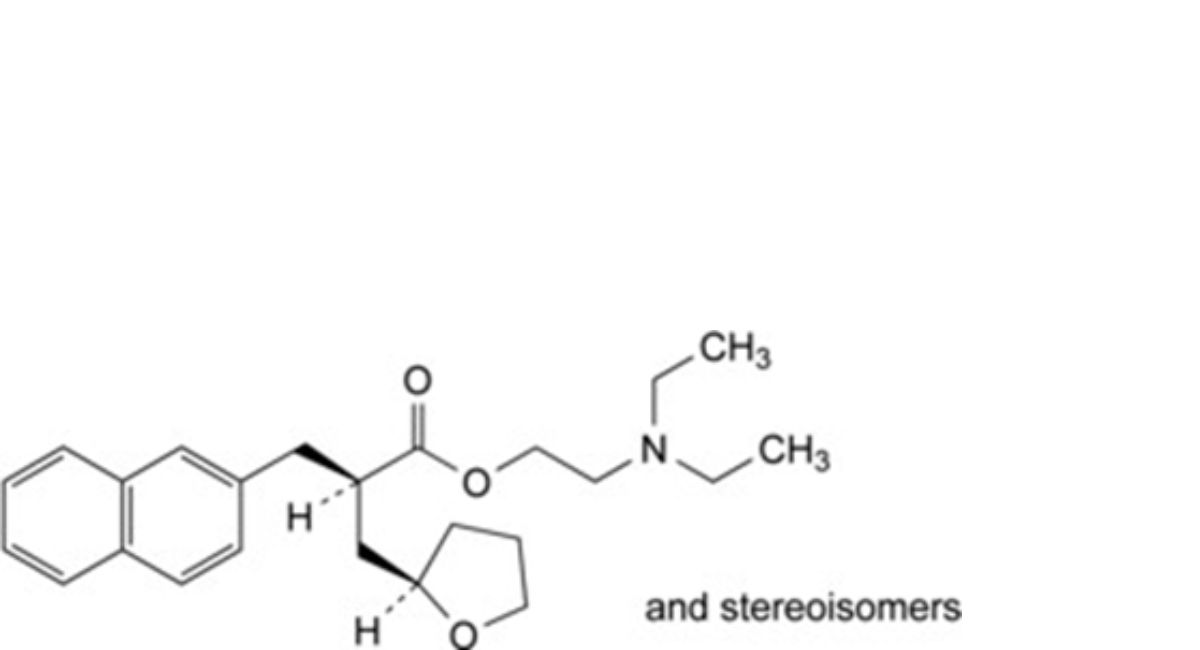
F. 2-(diethylamino)ethyl 2-[(naphthalen-2-yl)methyl]-3-(tetrahydrofuran-2-yl)propanoate.
Ph Eur