Action and use
Antihelminthic; ectoparasiticide
DEFINITION
Moxidectin Injection is a sterile solution of Moxidectin. It is supplied as a ready-to-use solution.
The injection complies with the requirements stated under Parenteral Preparations and with the following requirements.
Content of moxidectin, C37H53NO8
90.0 to 110.0% of the stated amount.
IDENTIFICATION
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in methanol.
(1) Dilute a volume of the injection to produce a solution containing 0.04% w/v of Moxidectin.
(2) 0.04% w/v of moxidectin BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel (Merck silica gel 60 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 5 μL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air, spray with anisaldehyde solution R1, heat at 105° for 5 to 10 minutes and allow to cool.
MOBILE PHASE
8 volumes of a 15% w/v solution of ammonium acetate adjusted to pH 9.6 with ammonia, 19 volumes of propan-2-ol and 43 volumes of ethyl acetate.
CONFIRMATION
The principal spot in the chromatogram obtained with solution (1) corresponds in position, colour and size to that in the chromatogram obtained with solution (2).
B. In the Assay, the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the principal peak in the chromatogram obtained with solution (2).
TESTS
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute the injection, if necessary, with water to produce a solution containing of 0.1% w/v moxidectin. To 2 mL of this solution, add 20 mL of 0.5% w/v of sodium chloride and 15 mL of dichloromethane and mix thoroughly. Centrifuge the mixture and separate the two layers. Repeat the extraction of the upper aqueous layer plus any suspended solids with a further 5 mL of dichloromethane. To the combined dichloromethane layers, add approximately 1 g of sodium sulfate, shake and filter. Dilute the filtrate to 25 mL with dichloromethane.
Prepare a solid phase extraction cartridge containing a silica sorbent (Varian Mega Bond Elut cartridges are suitable) by passing 50 mL of acetonitrile (followed by 20 mL of dichloromethane) through the cartridge under gravity. Pass 2 mL of the diluted filtrate through the cartridge under vacuum (followed by 20 mL of dichloromethane) discarding the eluent. Pass 6 mL of acetonitrile through the cartridge under gravity and collect the eluent. Force through any residual acetonitrile under vacuum. Dilute the eluent to 5 mL with acetonitrile.
(2) Dilute 1 volume of solution (1) to 100 volumes with acetonitrile.
(3) 0.25% w/v solution of moxidectin for system suitability EPCRS in acetonitrile.
(4) Dilute 1 volume of solution (2) to 10 volumes with acetonitrile.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 3.9 mm) packed with end-capped octadecylsilyl silica gel for chromatography (4 μm) (Novapak C18 is suitable).
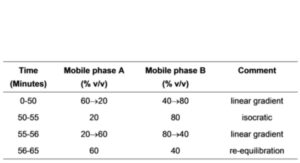
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 2.5 mL per minute.
(d) Use a column temperature of 50°
(e) Use a detection wavelength of 242 nm.
(f) Inject 10 μL of each solution.
MOBILE PHASE
mobile phase A: 0.7% w/v solution of ammonium acetate adjusted to pH 6.0 with either glacial acetic acid or ammonium hydroxide.
mobile phase B: acetonitrile.
When the chromatograms are recorded under the prescribed conditions, the relative retentions with reference to moxidectin (retention time about 29 minutes) of Impurity D is about 0.98.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the peak-to-valley ratio is at least 2.0 where Hp is the height above the baseline of the peak due to impurity D and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to moxidectin.
LIMITS
In the chromatogram obtained with solution (1):
the area of any other secondary peak is not greater than the area of principal peak in the chromatogram obtained with solution (2) (1%);
the sum of the areas of all the secondary peaks is not greater than 7 times the area of the principal peak in the chromatogram obtained with solution (2) (7%);
disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (4) (0.1%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in acetonitrile.
(1) Dilute a volume of the injection to produce a solution containing 0.1% w/v of Moxidectin. If the solution is cloudy, shake, allow to settle and use the supernatant.
(2) 0.1% w/v of moxidectin BPCRS.
(3) 0.1% w/v of moxidectin for system suitability EPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 3.9 mm) packed with end-capped octadecylsilyl silica gel for chromatography (4 μm) (Novapak C18 is suitable) fitted with a guard column (1.5 cm × 3.2 mm RP-18 (7 μm) is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2.5 mL per minute.
(d) Use a column temperature of 50°.
(e) Use a detection wavelength of 242 nm.
(f) Inject 10 μL of each solution.
MOBILE PHASE
40 volumes of a 1.925% w/v solution of ammonium acetate in water, adjusted to pH 4.8 with glacial acetic acid, and 60 volumes of acetonitrile.
When the chromatograms are recorded under the prescribed conditions, the relative retention with reference to moxidectin (retention time about 12 minutes) of impurity D is about 0.94.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the peak-to-valley ratio is at least 3.0 where Hp is the height above the baseline of the peak due to impurity D and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to moxidectin.
DETERMINATION OF CONTENT
Calculate the content of C37H53NO8 in the injection using the declared content of C37H53NO8 in moxidectin BPCRS.
STORAGE
Moxidectin Injection should be protected from light
IMPURITIES
The impurities limited by the requirements of this monograph include those listed under Moxidectin.






