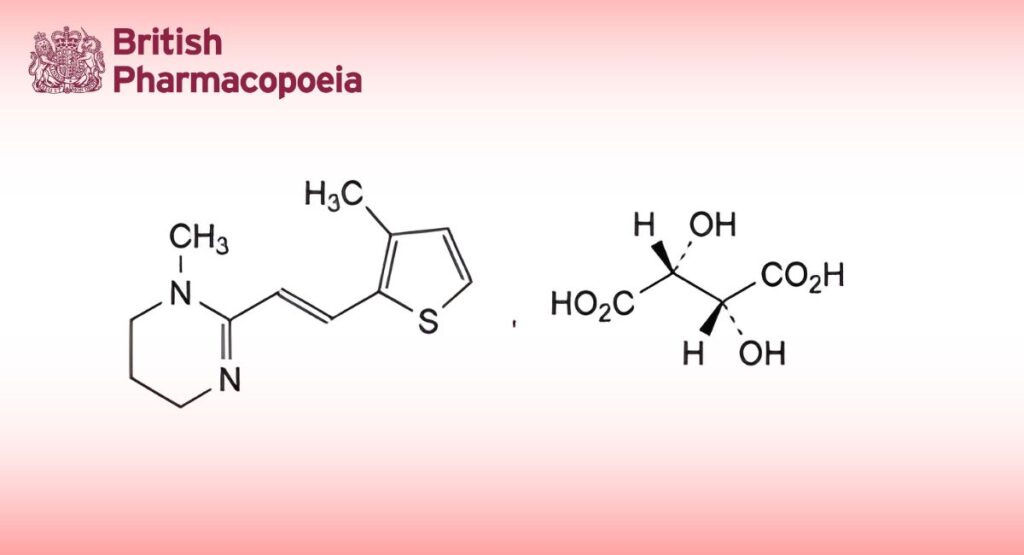
(Morantel Hydrogen Tartate for Veterinary Use, Ph. Eur. monograph 1546)
C16H22N2O6S 370.4 26155-31-7
Action and use
Antihelminthic.
DEFINITION
1-Methyl-2-[(1E)-2-(3-methylthiophen-2-yl)ethen-1-yl]-1,4,5,6-tetrahydropyrimidine hydrogen (2R,3R)-2,3- dihydroxybutanedioate.
Content
98.5 per cent to 101.5 per cent (dried substance).
CHARACTERS
Appearance
White or pale yellow, crystalline powder.
Solubility
Very soluble in water and in ethanol (96 per cent), practically insoluble in ethyl acetate.
IDENTIFICATION
First identification: B.
Second identification: A, C, D.\
A. Melting point (2.2.14): 167 °C to 172 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: morantel hydrogen tartrate CRS.
C. Dissolve about 10 mg in 1 mL of a 5 g/L solution of ammonium vanadate R. Evaporate to dryness. Add 0.1 mL of sulfuric acid R. A purple colour is produced.
D. Dissolve about 10 mg in 1 mL of a 4 g/L solution of sodium hydroxide R. Transfer to a separating funnel and shake with 5 mL of methylene chloride R. Discard the lower layer. Neutralise the upper layer with a few drops of dilute hydrochloric acid R. The solution gives reaction (b) of tartrates (2.3.1).
TESTS
Solution S
Dissolve 0.25 g in carbon dioxide-free water R and dilute to 25.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution GY6 or Y6 (2.2.2, Method II).
pH (2.2.3)
3.3 to 3.9 for solution S.
Related substances
Liquid chromatography (2.2.29). Carry out the test protected from light.
Test solution: Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase.
Reference solution (b): Dilute 1.0 mL of reference solution (a) to 10.0 mL with the mobile phase.
Reference solution (c): Expose 10 mL of reference solution (a) to daylight for 15 min before injection.
Reference solution (d): Dissolve 15 mg of tartaric acid R in the mobile phase and dilute to 100 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: base-deactivated end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: To a mixture of 0.35 volumes of triethylamine R and 85 volumes of water for chromatography R adjusted to pH 2.5 with phosphoric acid R, add 5 volumes of tetrahydrofuran R and 10 volumes of methanol R1.
Flow rate: 0.75 mL/min.
Detection: Spectrophotometer at 226 nm.
Injection: 20 μL.
Identification of impurities: Use the chromatogram obtained with reference solution (d) to identify the peak due to tartaric acid, use the chromatogram obtained with reference solution (c) to identify the peak due to the (Z)-isomer.
Relative retention: With reference to morantel (retention time = about 9.4 min): tartaric acid = about 0.4; (Z)-isomer = about 0.8.
Run time: Twice the retention time of morantel.
System suitability: Reference solution (c):
— resolution: minimum 2.0 between the principal peak and the preceding peak ((Z)-isomer).
Limits:
— any impurity apart from the peak due to tartaric acid: not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent);
— total: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (1.0 per cent);
— disregard limit: the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent).
Loss on drying (2.2.32)
Maximum 1.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.280 g in 40 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 37.04 mg of C16H22N2O6S.
STORAGE
Protected from light.
IMPURITIES
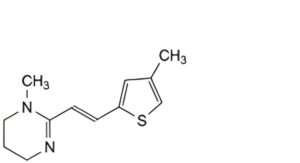
A. 1-methyl-2-[(1E)-2-(4-methylthiophen-2-yl)ethen-1-yl]-1,4,5,6-tetrahydropyrimidine,
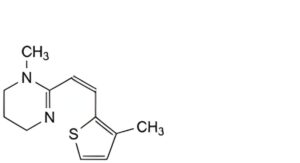
B. 1-methyl-2-[(1Z)-2-(3-methylthiophen-2-yl)ethen-1-yl]-1,4,5,6-tetrahydropyrimidine,
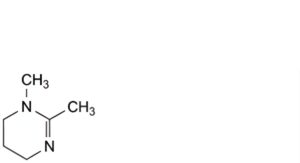
C. 1,2-dimethyl-1,4,5,6-tetrahydropyrimidine,
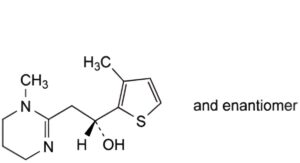
D. (1RS)-2-(1-methyl-1,4,5,6-tetrahydropyrimidin-2-yl)-1-(3-methylthiophen-2- yl)ethan-1-ol,
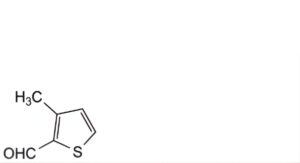
E. 3-methylthiophene-2-carbaldehyde.