Edition: BP 2025 (Ph. Eur. 11.6 update)
Methyldopa
General Notices
(Ph. Eur. monograph 0045)
C10H13NO4,1½H2O 238.2 41372-08-1
Action and use
Alpha2-adrenoceptor agonist; treatment of hypertension.
Preparation
Methyldopa Tablets
Ph Eur
DEFINITION
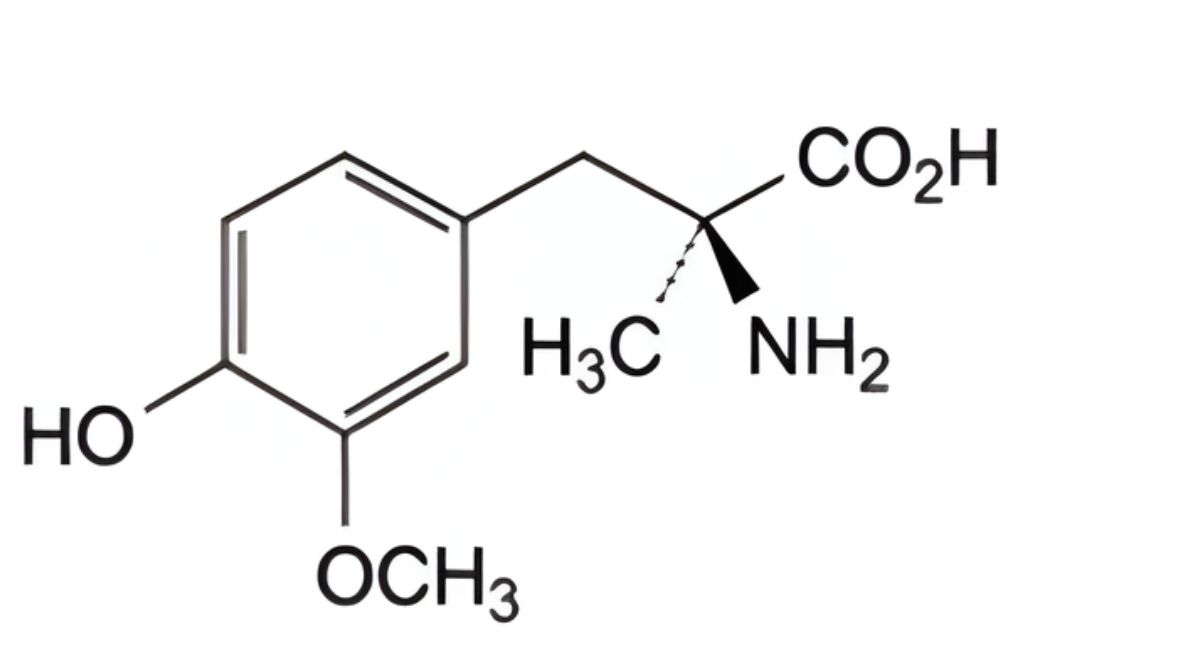
(2S)-2-Amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid sesquihydrate (L-methyldopa sesquihydrate).
Content
99.0 per cent to 101.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or yellowish-white, crystalline powder or colourless or almost colourless crystals.
Solubility
Slightly soluble in water, very slightly soluble in ethanol (96 per cent). It is freely soluble in dilute mineral acids.
IDENTIFICATION
Carry out either tests A, B or tests A, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison methyldopa CRS.
B. Enantiomeric purity (see Tests).
C. Specific optical rotation (2.2.7): -28.0 to -25.0.
Dissolve a quantity equivalent to 2.20 g of the anhydrous substance in aluminium chloride solution R and dilute to 50.0 mL with the same solution.
TESTS
Appearance of solution
Dissolve 1.0 g in 1 M hydrochloric acid and dilute to 25 mL with the same solvent. The solution is not more intensely coloured than reference solution BY6 or B6 (2.2.2, Method II).
Acidity
Dissolve 1.0 g with heating in 100 mL of carbon dioxide-free water R. Add 0.1 mL of methyl red solution R. Not more than 0.5 mL of 0.1 M sodium hydroxide is required to produce the pure yellow colour of the indicator.
Absorbance (2.2.25)
Test solution Dissolve 40.0 mg in 0.1 M hydrochloric acid and dilute to 100.0 mL with the same acid. Dilute 10.0 mL of the solution to 100.0 mL with 0.1 M hydrochloric acid.
Spectral range 230-350 nm.
Absorption maximum At 280 nm.
Specific absorbance at the absorption maximum 122 to 137 (anhydrous substance).
Enantiomeric purity
Liquid chromatography (2.2.29).
Test solution Dissolve 25 mg of the substance to be examined in the mobile phase and dilute to 25.0 mL with the mobile phase.
Reference solution (a) Dilute 5.0 mL of the test solution to 20.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 50.0 mL with the mobile phase.
Reference solution (b) Dissolve 2 mg of racemic methyldopa CRS in the mobile phase and dilute to 10.0 mL with the mobile phase.
Column:
— size: l = 0.15 m, Ø = 3.9 mm;
— stationary phase: spherical end-capped octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase Dissolve separately 0.200 g of copper acetate R and 0.387 g of N,N-dimethyl-L phenylalanine R in water R; mix the 2 solutions and adjust immediately to pH 4.3 with acetic acid R; add 50 mL of methanol R and dilute to 1000 mL with water R; mix and filter.
Equilibrate the column with the mobile phase for about 2 h.
If necessary, decrease the concentration of methanol R so the peak corresponding to D-methyldopa is clearly separated from the negative system peak that appears at about 6 min.
Flow rate 1 mL/min.
Detection Spectrophotometer at 280 nm.
Injection 20 µL.
Run time Twice the retention time of L-methyldopa.
Relative retention With reference to L-methyldopa (retention time = about 14 min): D methyldopa = about 0.7.
System suitability Reference solution (b):
— resolution: minimum 5.0 between the peaks due to D-methyldopa and L-methyldopa. Limit:
— D-methyldopa (impurity D): not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent).
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Test solution Dissolve 0.100 g of the substance to be examined in 0.1 M hydrochloric acid and dilute to 25.0 mL with the same acid.
Reference solution (a) Dilute 1.0 mL of the test solution to 50.0 mL with 0.1 M hydrochloric acid. Dilute 5.0 mL of this solution to 100.0 mL with 0.1 M hydrochloric acid.
Reference solution (b) Dissolve the contents of a vial of methyldopa for system suitability CRS (containing impurities A, B and C) in 1.0 mL of 0.1 M hydrochloric acid.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: spherical di-isobutyloctadecylsilyl silica gel for chromatography R (5 µm) with a pore size of 8 nm.
Mobile phase methanol R, 0.1 M phosphate buffer solution pH 3.0 R (15:85 V/V). Flow rate 1 mL/min.
Detection Spectrophotometer at 280 nm.
Injection 20 µL.
Run time 6 times the retention time of methyldopa.
Identification of impurities Use the chromatogram supplied with methyldopa for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A, B and C.
Relative retention With reference to methyldopa (retention time = about 5 min): impurity A = about 1.9; impurity B = about 4.3; impurity C = about 4.9.
System suitability Reference solution (b):
— resolution: minimum 2.0 between the peaks due to impurities B and C.
Limits:
— correction factors: for the calculation of content, multiply the peak areas of the following impurities by the corresponding correction factor: impurity B = 2.6; impurity C = 1.3;
— impurities A, B, C: for each impurity, not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.15 per cent);
— unspecified impurities: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent);
— total: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent);
— disregard limit: 0.3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.03 per cent).
Water (2.5.12)
10.0 per cent to 13.0 per cent, determined on 0.20 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.180 g, heating if necessary, in 50 mL of glacial acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 21.12 mg of C10H13NO4.
STORAGE
Protected from light.
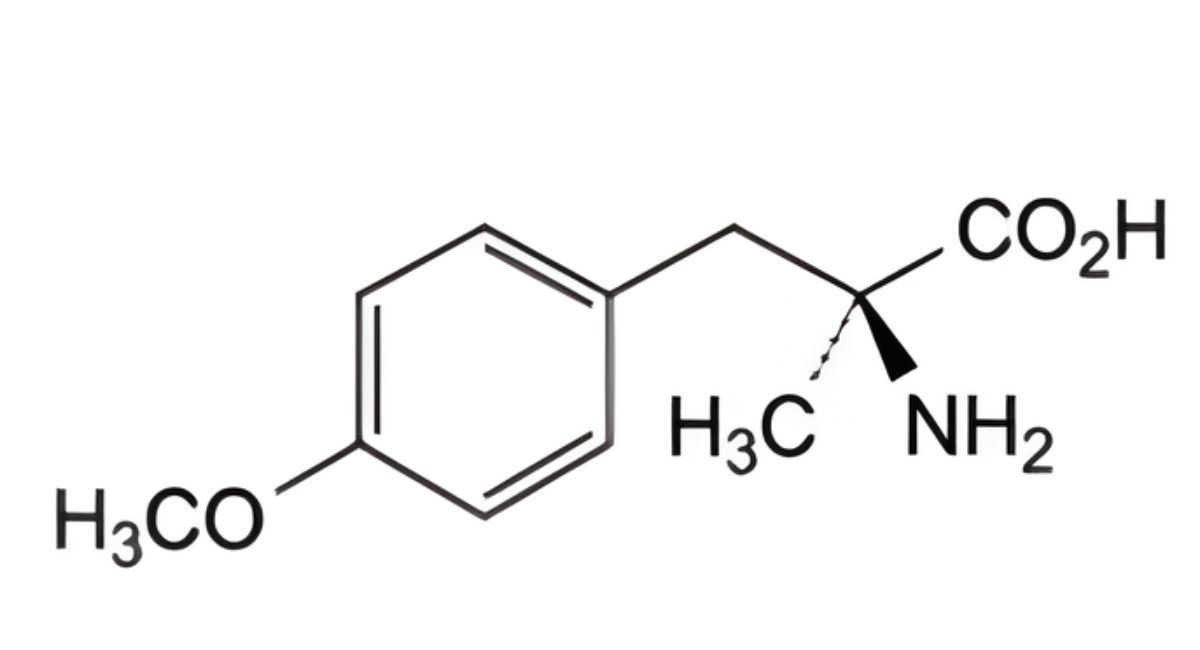
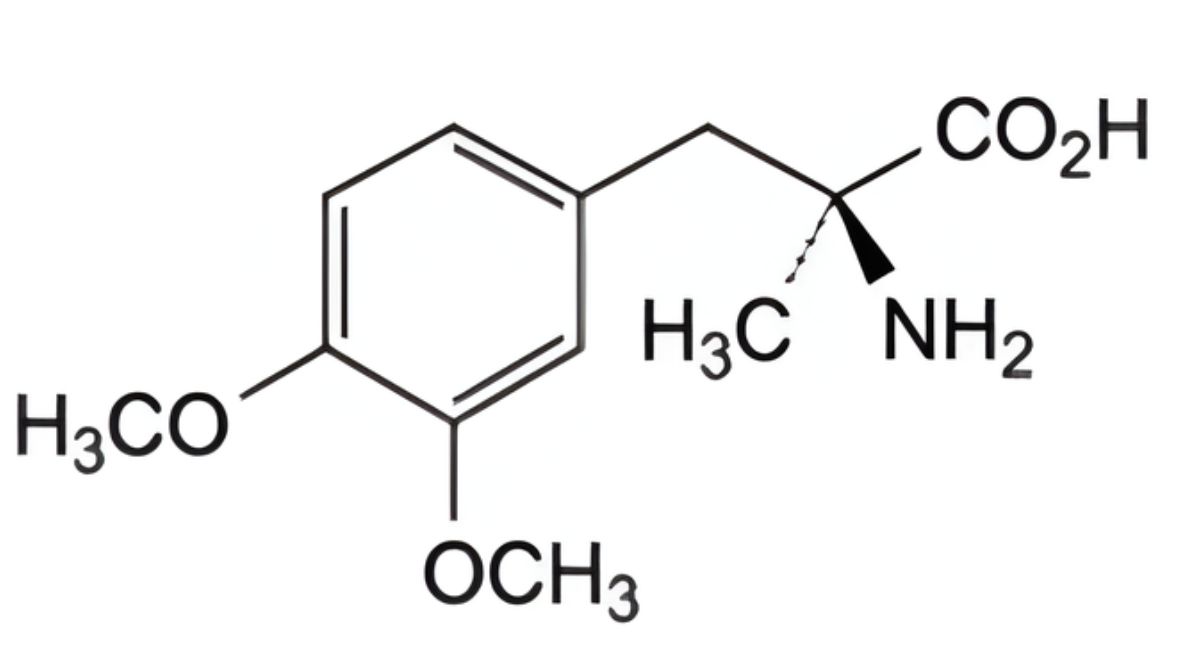
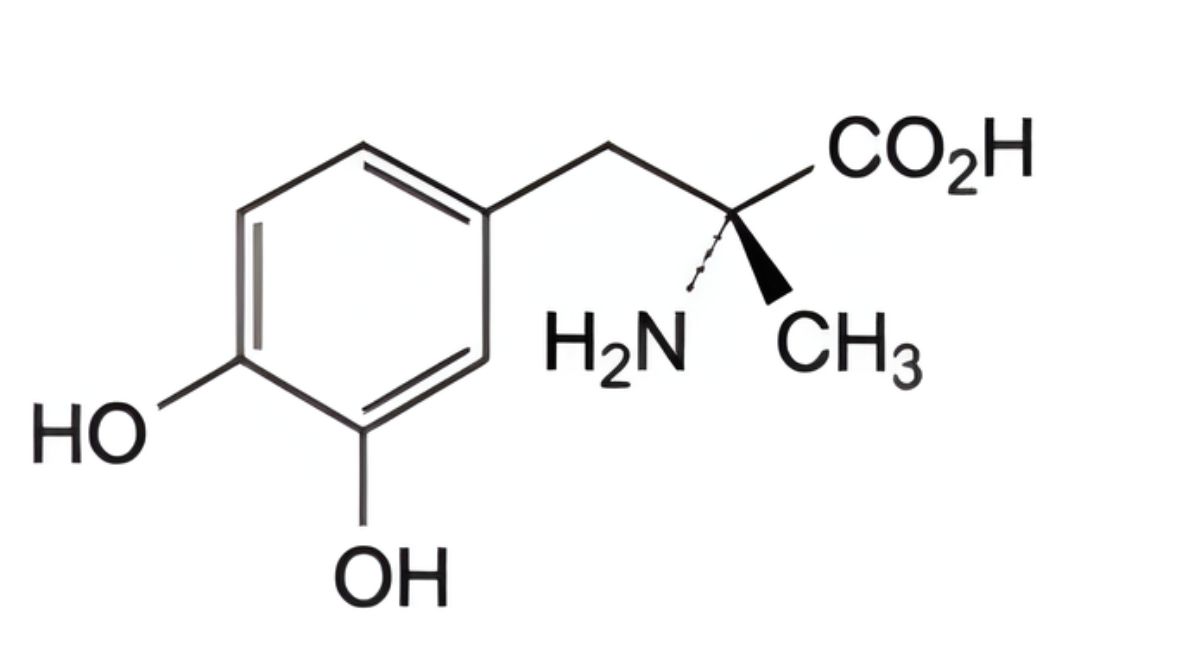
IMPURITIES
Specified impurities A, B, C, D.