Action and use
Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory.
DEFINITION
Meloxicam Injection is a sterile solution of Meloxicam in Water for Injections.
The injection complies with the requirements stated under Parenteral Preparations and with the following requirements.
Content of meloxicam, C14H13N3O4S2
95.0 to 105.0% of the stated amount.
IDENTIFICATION
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
(1) Dilute a volume of the injection containing 10 mg of Meloxicam to 20 mL with acetone, stir for 15 minutes, filter and use the filtrate.
(2) Dissolve 10 mg of meloxicam BPCRS in 10 mL of acetone, add 2 mL of water and dilute to 20 mL with acetone.
CHROMATOGRAPHIC CONDITIONS
(a) Use a silica gel F254 plate (Merck HPTLC plates are suitable).
(b) Use the mobile phase described below.
(c) Apply 20 μL of each solution.
(d) Develop the plate to 8 cm.
(e) After removal of the plate, dry in air and examine under ultraviolet light (254 nm).
MOBILE PHASE
1 volume of 13.5M ammonia, 20 volumes of methanol and 80 volumes of dichloromethane.
CONFIRMATION
The principal spot in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
B. In the Assay, the retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to that of the principal peak in the chromatogram obtained with solution (2).
TESTS
Alkalinity
pH, 7.5 to 9.1, Appendix V L.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Add 0.3 mL of 0.4M sodium hydroxide to a volume of the injection containing 40 mg of Meloxicam and dilute with methanol (40%) to produce 10 mL.
(2) Dilute 1 mL of solution (1) to 100 mL with methanol (40%).
(3) Add 0.3 mL of 0.4M sodium hydroxide to 40 mg of meloxicam impurity standard
BPCRS and dilute with methanol
(40%) to produce 10 mL.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (10 cm × 4.0 mm) packed with octadecylsilyl silica gel for chromatography (10 μm) (Kromasil 100 C18 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use an ambient column temperature.
(e) Use detection wavelengths of 260 nm and 350 nm.
(f) Inject 10 μL of each solution.
MOBILE PHASE
Mobile phase A: 0.1% w/v solution of potassium dihydrogen orthophosphate adjusted to pH 6.0 with 2M sodium hydroxide.
Mobile phase B: methanol.
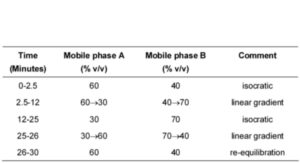
SYSTEM SUITABILITY
The chromatogram obtained with solution (3):
resembles that supplied with meloxicam impurity standard BPCRS at 260 nm and 350 nm and;
the resolution between the peaks due to meloxicam and impurity A at 350 nm is at least 3.0;
the resolution between the peaks due to impurity B and meloxicam at 260 nm is at least 3.0.
LIMITS
Identify any peaks in the chromatogram obtained with solution (1) corresponding to impurity A and impurity B using the chromatogram obtained with solution (3) and the chromatogram supplied with meloxicam impurity standard BPCRS.
In the chromatogram obtained with solution (1):
identify any peak corresponding to impurity A at 350 nm and multiply the area of this peak by a correction factor of 2.0;
the area of any peak corresponding to impurity B at 260 nm is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) at 350 nm (2.0%);
the area of any peak corresponding to impurity A at 350 nm is not greater than the area of the principal peak in the chromatogram obtained with solution (2) at 350 nm (1.0%).
the area of any other secondary peak, at the wavelength giving the higher value for the impurity, is not greater than the area of the peak in the chromatogram obtained with solution (2) at the same wavelength (1.0%).
The nominal total content of any such impurities is not greater than 3.5%.
Disregard any peak with an area less than 0.3 times the area of the principal peak in the chromatogram obtained with solution (2) at the same wavelength (0.3%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) To a volume of the injection containing 40 mg of Meloxicam add 0.3 mL of 0.4M sodium hydroxide and add sufficient methanol (40%) to produce 10 mL. Dilute 1 mL of the resulting solution to 10 mL with methanol (40%).
(2) 0.04% w/v of meloxicam BPCRS in methanol (40%).
(3) Add 0.3 mL of 0.4M sodium hydroxide to 40 mg of meloxicam impurity standard BPCRS and dilute with methanol (40%) to produce 10 mL.
CHROMATOGRAPHIC CONDITIONS
The chromatographic procedure described under Related substances may be used but with a detection wavelength of 350 nm.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (3):
closely resembles the chromatogram supplied with meloxicam impurity standard BPCRS at 350 nm;
the resolution between the peaks due to meloxicam and impurity A at 350 nm is at least 3.0.
DETERMINATION OF CONTENT
Calculate the content of C14H13N3O4S2 using the declared content of C14H13N3O4S2 in meloxicam BPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include those listed under Meloxicam.






