Marek’s Disease Vaccine (Turkey Herpes Virus)
Marek’s Disease Vaccine, Living (HVT)
(Marek’s Disease Vaccine (Live), Ph. Eur. monograph 0589)
1 DEFINITION
Marek’s disease vaccine (live) is a preparation of a suitable strain or strains of Marek’s disease virus (gallid herpesvirus 2 or 3) and/or turkey herpesvirus (meleagrid herpesvirus 1). This monograph applies to vaccines intended for administration to chickens and/or chicken embryos for active immunisation.
2 PRODUCTION
2-1 PREPARATION OF THE VACCINE
The vaccine virus is grown in cell cultures. If the vaccine contains more than one type of virus, the different types are grown separately. The vaccine may be freeze-dried or stored in liquid nitrogen.
2-2 SUBSTRATE FOR VIRUS PROPAGATION
2-2-1 Cell cultures
The cell cultures comply with the requirements for cell cultures for the production of vaccines for the veterinary use (5.2.4).
2-3 CHOICE OF VACCINE VIRUS
The vaccine virus shall be shown to be satisfactory with respect to safety (5.2.6) and efficacy (5.2.7) for the chickens and/or chicken embryos for which it is intended.
The tests shown below for residual pathogenicity of the strain (section 2-3-1-1), increase in virulence (section 2-3-2) and immunogenicity (section 2-3-3) may be used during the demonstration of safety and efficacy. Additional testing may be needed to demonstrate safety in breeds of chickens known to be particularly susceptible to Marek’s disease virus, unless the vaccine is to be contraindicated.
2-3-1 Safety
Marek’s Disease Vaccine, Living
2-3-1-1 Residual pathogenicity of the strain. Carry out the test for the route to be recommended for vaccination that is likely to be the least safe and in the category of chickens for which the vaccine is intended that is likely to be the most susceptible for Marek’s disease.
Carry out the test in chickens if the vaccine is intended for chickens; carry out the test in chicken embryos if the vaccine is intended for chicken embryos; carry out the test in chickens and in chicken embryos if the vaccine is intended for both.
Use vaccine virus at the least attenuated passage level that will be present between the master seed lot and a batch of the vaccine.
Vaccines intended for use in chickens Use not fewer than 80 1-day-old chickens from a flock free from specified pathogens (SPF) (5.2.2). Divide them randomly into 2 groups of not fewer than 40 chickens and maintain the groups separately. Administer by a suitable route to each chicken of one group (I) a quantity of the vaccine virus equivalent to not less than 10 times the maximum virus titre likely to be contained in 1 dose of the vaccine. Administer by a suitable route to each chicken of the other group (II) a quantity of virulent Marek’s disease virus that will cause mortality and/or severe macroscopic lesions of Marek’s disease in not fewer than 70 per cent of the effective number of chickens within 70 days (initial number reduced by the number that die within the first 7 days of the test).
Vaccines intended for use in chicken embryos Use not fewer than 150 embryonated eggs from an SPF flock (5.2.2). Divide them randomly into 3 groups of not fewer than 50 embryonated eggs and maintain the groups separately but under identical incubation conditions. Not later than the recommended day of vaccination, administer by the method to be recommended to each embryonated egg of one group (I) a quantity of the vaccine virus equivalent to not less than 10 times the maximum virus titre likely to be contained in 1 dose of the vaccine. Administer by a suitable route to each embryonated egg of another group (II) a quantity of virulent Marek’s disease virus that will cause mortality and/or severe macroscopic lesions of Marek’s disease in not fewer than 70 per cent of the effective number of hatched chickens within 70 days (initial number reduced by the number that die within the first 7 days after hatching). Keep the last group (III) non-inoculated. The test is not valid if there is a significant difference in hatchability between groups I and III and the hatchability in any of the 3 groups is less than 80 per cent.
Provided that the chickens and chicken embryos are derived from the same flock, a common control group for in ovo and parenteral administration can be used.
Irrespective of whether the vaccine was administered to chickens or chicken embryos, observe the chickens of group II at least daily for 70 days and those of group I at least daily for 120 days.
The test is not valid if one or more of the following apply:
— more than 10 per cent of the chickens in any of the 3 groups die within the first 7 days;
— fewer than 70 per cent of the effective number of chickens in group II show macroscopic lesions of Marek’s disease;
The vaccine virus complies with the test if:
— no chicken of group I shows notable clinical signs or macroscopic lesions of Marek’s disease or dies from causes attributable to the vaccine virus;
— at 120 days the number of surviving chickens of group I is not fewer than 80 per cent of the effective number.
2-3-2 Increase in virulence
The test for increase in virulence is required for Marek’s disease virus vaccine strains but not for turkey herpesvirus vaccine strains, which are naturally apathogenic.
Carry out the test according to general chapter 5.2.6.
Vaccines intended for use in chickens: Administer to each 1-day-old SPF chicken (5.2.2) by the intramuscular route a quantity of the vaccine virus that will allow recovery of virus for the passages described below.
Vaccines intended for use only in chicken embryos or intended for use in chickens and in chicken embryos: Administer to each embryonated egg not later than the recommended day for vaccination by the in ovo route, using the recommended method, a quantity of the vaccine virus that will allow recovery of virus for the passages described below.
If the properties of the vaccine virus allow sequential passage through 5 groups via natural spreading, this method may be used, otherwise passage as described below is carried out.
5-7 days after administering the vaccine to chickens or 5-7 days after hatching when the vaccine has been administered in ovo, prepare a suspension of white blood cells from each chicken and pool these samples. Administer a suitable volume of the pooled samples by the intraperitoneal route to each 1-day-old SPF chicken (5.2.2) of the next group. Carry out this passage operation not fewer than 4 times; verify the presence of the virus at each passage. If the virus is not found at a passage level, repeat the passage by administration to a group of 10 chickens. Carry out the test for residual pathogenicity (section 2-3-1-1) using the material used for the 1st passage and the virus at the final passage level. Administer the virus
by the route to be recommended for vaccination that is likely to be the least safe for use in these chickens or chicken embryos.
The vaccine virus complies with the test if no indication of increase in virulence of the virus recovered for the final passage compared with the material used for the 1st passage is observed. If virus is not recovered after an initial passage in 5 chickens or chicken embryos and a subsequent repeat passage in 10 chickens or chicken embryos, the vaccine virus also complies with the test.
2-3-3 Immunogenicity
A test is carried out for each route and method of administration to be recommended, using in each case chickens of the minimum age to be recommended for vaccination or chicken embryos. The quantity of the vaccine virus administered to each chicken or chicken embryo is not greater than the minimum virus titre to be stated on the label and the virus is at the most attenuated passage level that will be present in a batch of the vaccine.
Vaccines intended for use in chickens Use not fewer than 60 chickens of the same origin and from an SPF flock (5.2.2). Vaccinate by a route to be recommended not fewer than 30 chickens. Maintain not fewer than 30 chickens as controls.
Vaccines intended for use in chicken embryos Use embryonated chickens of the same origin and from an SPF flock (5.2.2). Vaccinate by the in ovo route using the method to be recommended, 50 per cent of the embryonated eggs. Maintain 50 per cent of the embryonated eggs as controls. The test is not valid if any group consists of fewer than 30 hatched chicks.
Irrespective of whether the vaccine was administered to chickens or chicken embryos, challenge each chicken not later than 9 days after vaccination by a suitable route with a sufficient quantity of virulent Marek’s disease virus. Observe the chickens at least daily for 70 days after challenge. Record the deaths and the number of surviving chickens that show clinical signs of disease. At the end of the observation period, euthanise all the surviving chickens and carry out an examination for macroscopic lesions of Marek’s disease.
The test is not valid if:
— during the observation period after challenge, fewer than 70 per cent of the control chickens die or show severe clinical signs or macroscopic lesions of Marek’s disease;
— and/or, during the period between the vaccination and challenge, more than 10 per cent of the control or vaccinated chickens show abnormal clinical signs or die from causes not attributable to the vaccine.
The vaccine virus complies with the test if the relative protection percentage, calculated using the following expression, is not less than 80 per cent:
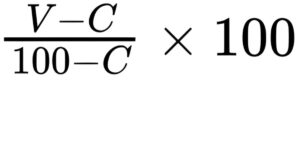
V = percentage of challenged vaccinated chickens that survive to the end of the observation period without notable clinical signs or macroscopic lesions of Marek’s disease;
C = percentage of challenged control chickens that survive to the end of the observation period without notable clinical signs or macroscopic lesions of Marek’s disease.
3 BATCH TESTS
3-1 Identification
The vaccine virus is identified using a suitable method, for example an immunostaining test in susceptible cell cultures using monoclonal antibodies, to demonstrate the presence of each type of virus stated on the label.
3-2 Bacteria and fungi
The vaccine, including where applicable the diluent supplied for reconstitution, complies with the test for sterility prescribed in the general monograph Vaccines for veterinary use (0062).
3-3 Mycoplasmas (2.6.7)
The vaccine complies with the test for mycoplasmas.
3-4 Extraneous agents (5.2.5)
The vaccine is free from extraneous agents.
3-5 Virus titre
3-5-1 Vaccines containing one type of virus. Titrate the vaccine virus by inoculation into suitable cell cultures (5.2.4). If the virus titre is determined in plaque-forming units (PFU), only primary plaques are taken into consideration. The vaccine complies with the test if one dose contains not less than the minimum virus titre stated on the label.
3-5-2 Vaccines containing more than one type of virus. For vaccines containing more than one type of virus, titrate each virus by inoculation into suitable cell cultures (5.2.4), reading the results by immunostaining using antibodies. The vaccine complies with the test if one dose contains for each vaccine virus not less than the minimum virus titre stated on the label.
3-6 Potency
The vaccine complies with the test for immunogenicity (section 2-3-3) when administered according to the recommended schedule by a recommended route and method. It is not necessary to carry out the potency test for each batch of the vaccine if it has been carried out on a representative batch using a vaccinating dose containing not more than the minimum virus titre stated on the label.






