Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 2162)
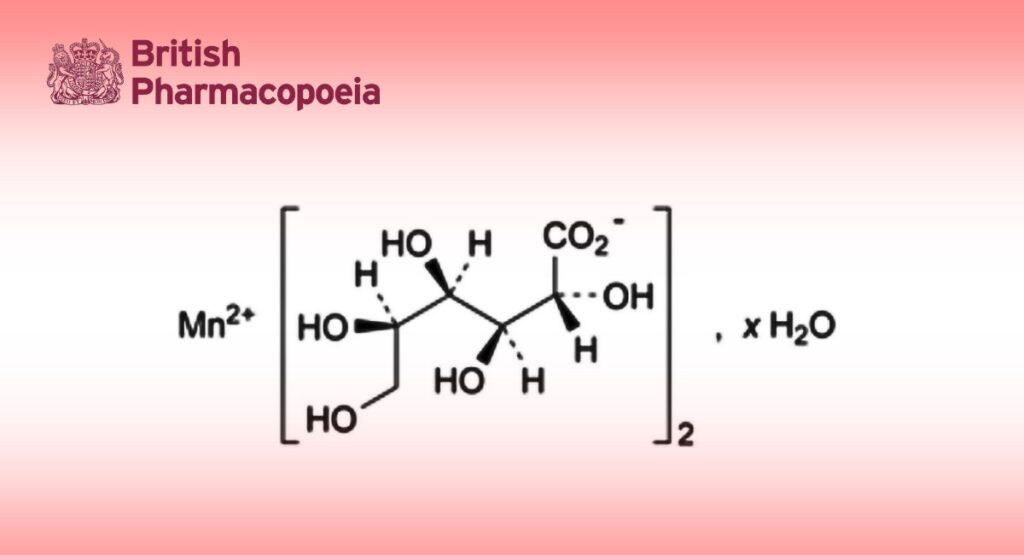
C12H22MnO14,xH2O 445.2 (anhydrous substance)
DEFINITION
Anhydrous or hydrated manganese(II) bis[(2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate] (anhydrous or hydrated manganese(II) di(D-gluconate)).
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or pale pink, slightly hygroscopic, crystalline powder.
Solubility
Soluble in water, practically insoluble in anhydrous ethanol, insoluble in methylene chloride.
IDENTIFICATION
A. Thin-layer chromatography (2.2.27).
Test solution Dissolve 20 mg of the substance to be examined in 1 mL of water R.
Reference solution Dissolve 20 mg of calcium gluconate CRS in 1 mL of water R, heating if necessary in a water-bath at 60 °C.
Plate TLC silica gel plate R (5-40 μm) [or TLC silica gel plate R (2-10 μm)].
Mobile phase concentrated ammonia R, ethyl acetate R, water R, ethanol (96 per cent) R (10:10:30:50 V/V/V/V).
Application 1 μL.
Development Over 3/4 of the plate.
Drying At 105 °C for 20 min, then allow to cool to room temperature.
Detection Spray with a solution containing 25 g/L of ammonium molybdate R and 10 g/L of cerium sulfate R in dilute sulfuric acid R, and heat at 105 °C for about 10 min.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
B. Dissolve 50 mg in 5 mL of water R. Add 0.5 mL of ammonium sulfide solution R. A pale pink precipitate is formed that dissolves upon the addition of 1 mL of glacial acetic acid R.
TESTS
Solution S
Dissolve 1.0 g in water R and dilute to 50 mL with the same solvent.
Appearance of solution
Solution S is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than intensity 6 of the range of reference solutions of the most appropriate colour (2.2.2, Method II).
Sucrose and reducing sugars
Dissolve 0.5 g in a mixture of 2 mL of hydrochloric acid R1 and 10 mL of water R. Boil for 5 min, allow to cool, add 10 mL of sodium carbonate solution R and allow to stand for 10 min.
Dilute to 25 mL with water R and filter. To 5 mL of the filtrate add 2 mL of cupri-tartaric solution R and boil for 1 min. Allow to stand for 2 min. No red precipitate is formed.
Chlorides (2.4.4)
Maximum 500 ppm.
Dilute 5 mL of solution S to 15 mL with water R.
Sulfates (2.4.13)
Maximum 500 ppm.
Dissolve 2.0 g in a mixture of 10 mL of acetic acid R and 90 mL of distilled water R.
Zinc
Maximum 50 ppm.
To 10 mL of solution S add 1 mL of sulfuric acid R and 0.1 mL of potassium ferrocyanide solution R. After 30 s, any opalescence in the solution is not more intense than that in a mixture of 1.0 mL of zinc standard solution (10 ppm Zn) R, 9 mL of water R, 1 mL of sulfuric acid R and 0.1 mL of potassium ferrocyanide solution R.
Water (2.5.32)
Maximum 9.0 per cent, determined on 80 mg.
Microbial contamination
TAMC: acceptance criterion 10 CFU/g (2.6.12).
TYMC: acceptance criterion 10 CFU/g (2.6.12).
ASSAY
Dissolve 0.400 g in 50 mL of water R. Add 10 mg of ascorbic acid R, 20 mL of ammonium chloride buffer solution pH 10.0 R and 0.2 mL of a 2 g/L solution of mordant black 11 R in triethanolamine R. Titrate with 0.1 M sodium edetate until the colour changes from violet to pure blue.
1 mL of 0.1 M sodium edetate is equivalent to 44.52 mg of C12H22MnO14.
STORAGE
In a non-metallic, airtight container.