Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 1235)
C12H24O11 344.3 585-88-6
Action and use
Sweetening agent.
DEFINITION
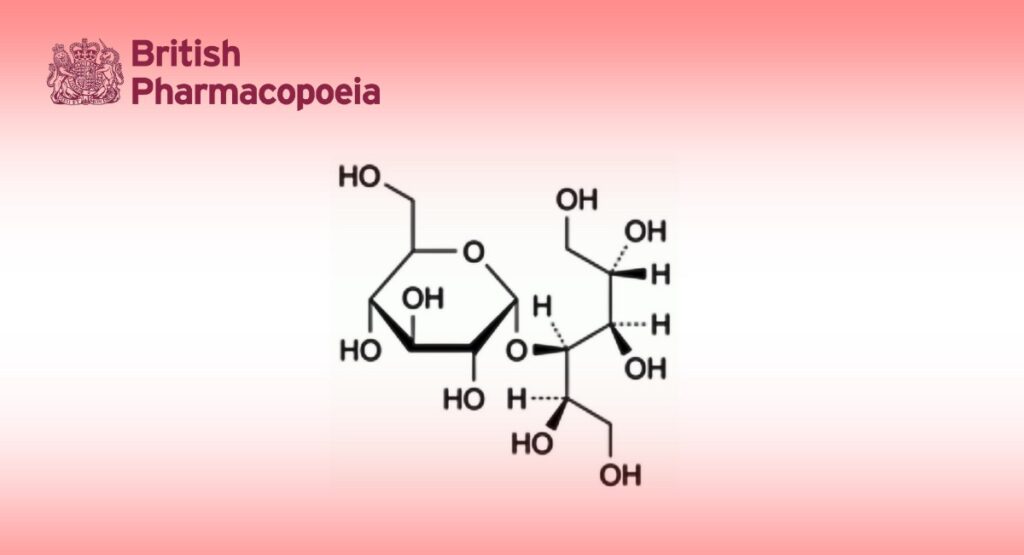
4-O-α-D-Glucopyranosyl-D-glucitol (D-maltitol).
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Very soluble in water, practically insoluble in anhydrous ethanol.
IDENTIFICATION
First identification: A.
Second identification: B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison maltitol CRS.
B. Melting point (2.2.14): 148 °C to 151 °C.
C. Specific optical rotation (2.2.7): + 105.5 to + 108.5 (anhydrous substance).
Dissolve 5.00 g in water R and dilute to 100.0 mL with the same solvent.
D. Thin-layer chromatography (2.2.27).
Test solution Dissolve 25 mg of the substance to be examined in water R and dilute to 10 mL with the same solvent.
Reference solution (a) Dissolve 25 mg of maltitol CRS in water R and dilute to 10 mL with the same solvent.
Reference solution (b) Dissolve 25 mg of maltitol CRS and 25 mg of sorbitol CRS in water R and dilute to 10 mL with the same solvent.
Plate TLC silica gel G plate R.
Mobile phase water R, ethyl acetate R, propanol R (10:20:70 V/V/V).
Application 2 μL.
Development Over 3/4 of the plate.
Drying In air.
Detection Spray with 4-aminobenzoic acid solution R. Dry in a current of cold air until the acetone is removed. Heat at 100-105 °C for 15 min. Allow to cool and spray with a 2 g/L solution of sodium periodate R.
Dry in a current of cold air. Heat at 100 °C for 15 min.
System suitability Reference solution (b):
— the chromatogram shows 2 clearly separated spots.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
TESTS
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Dissolve 5.0 g in water R and dilute to 50 mL with the same solvent.
Conductivity (2.2.38)
Maximum 20 μS·cm .
Dissolve 20.0 g in carbon dioxide-free water R prepared from distilled water R and dilute to 100.0 mL with the same solvent. Measure the conductivity of the solution, while gently stirring with a magnetic stirrer.
Reducing sugars
Maximum 0.2 per cent, expressed as glucose equivalent.
Dissolve 5.0 g in 6 mL of water R with the aid of gentle heat. Cool and add 20 mL of cupri-citric solution R and a few glass beads. Heat so that boiling begins after 4 min and maintain boiling for 3 min. Cool rapidly and add 100 mL of a 2.4 per cent V/V solution of glacial acetic acid R and 20.0 mL of 0.025 M iodine. With continuous shaking, add 25 mL of a mixture of 6 volumes of hydrochloric acid R and 94 volumes of water R and, when the precipitate has dissolved, titrate the excess of iodine with 0.05 M sodium thiosulfate using 1 mL of starch solution R, added towards the end of the titration as indicator. Not less than 12.8 mL of
0.05 M sodium thiosulfate is required.
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 5.0 g of the substance to be examined in 20 mL of water R and dilute to 100.0 mL with the same solvent.
Reference solution (a) Dissolve 0.50 g of maltitol CRS in 2.0 mL of water R and dilute to 10.0 mL with the same solvent.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with water R.
Reference solution (c) Dilute 10.0 mL of reference solution (b) to 100.0 mL with water R.
Reference solution (d) Dissolve 0.5 g of maltitol R and 0.5 g of sorbitol R in 5 mL of water R and dilute to 10 mL with the same solvent.
Column:
— size: l = 0.3 m, Ø = 7.8 mm;
— stationary phase: strong cation-exchange resin (calcium form) R (9 μm);
— temperature: 85 ± 1 °C.
Mobile phase Degassed water for chromatography R.
Flow rate 0.5 mL/min.
Detection Differential refractometer maintained at a constant temperature (e.g. 35 °C).
Injection 20 μL of the test solution and reference solutions (b), (c) and (d).
Run time 3 times the retention time of maltitol.
Relative retention With reference to maltitol (retention time = about 16 min): impurity B = about 0.8; impurity A = about 1.8.
System suitability Reference solution (d):
— resolution: minimum 2.0 between the peaks due to maltitol and impurity A.
Limits:
— any impurity: for each impurity, not more than the area of the principal peak in the chromatogram
obtained with reference solution (b) (1.0 per cent);
— total: not more than twice the area of the principal peak in the chromatogram obtained with reference
solution (b) (2.0 per cent);
— disregard limit: the area of the principal peak in the chromatogram obtained with reference
solution (c) (0.1 per cent).
Water (2.5.12)
Maximum 1.0 per cent, determined on 1.00 g.
Microbial contamination
If intended for use in the manufacture of parenteral preparations:
— TAMC: acceptance criterion: 10 CFU/g (2.6.12).
If not intended for use in the manufacture of parenteral preparations:
— TAMC: acceptance criterion 10 CFU/g (2.6.12);
— TYMC: acceptance criterion 10 CFU/g (2.6.12);
— absence of Escherichia coli (2.6.13);
— absence of Salmonella (2.6.13).
Bacterial endotoxins (2.6.14)
If intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins:
— less than 4 IU/g for parenteral preparations having a concentration of less than 100 g/L of maltitol;
— less than 2.5 IU/g for parenteral preparations having a concentration of 100 g/L or more of maltitol.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection Test solution and reference solution (a).
Calculate the percentage content of C12H24O11 taking into account the assigned content of maltitol CRS.
LABELLING
The label states:
— where applicable, the maximum concentration of bacterial endotoxins;
— where applicable, that the substance is suitable for use in the manufacture of parenteral
preparations.
IMPURITIES
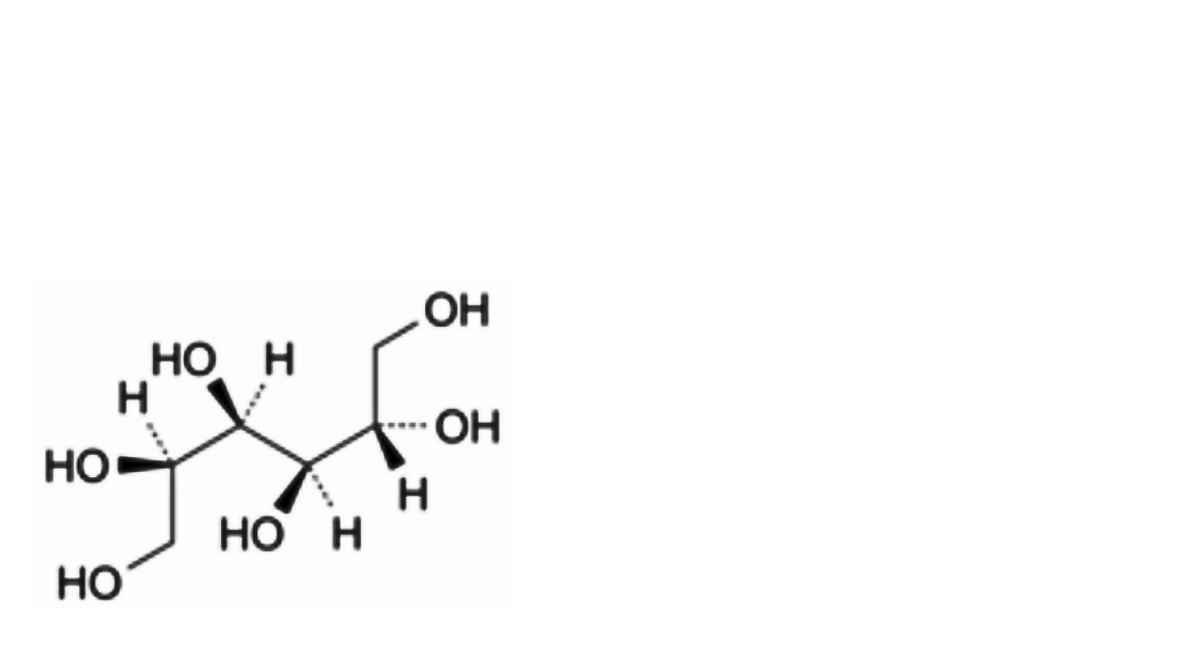
A. D-glucitol (D-sorbitol),
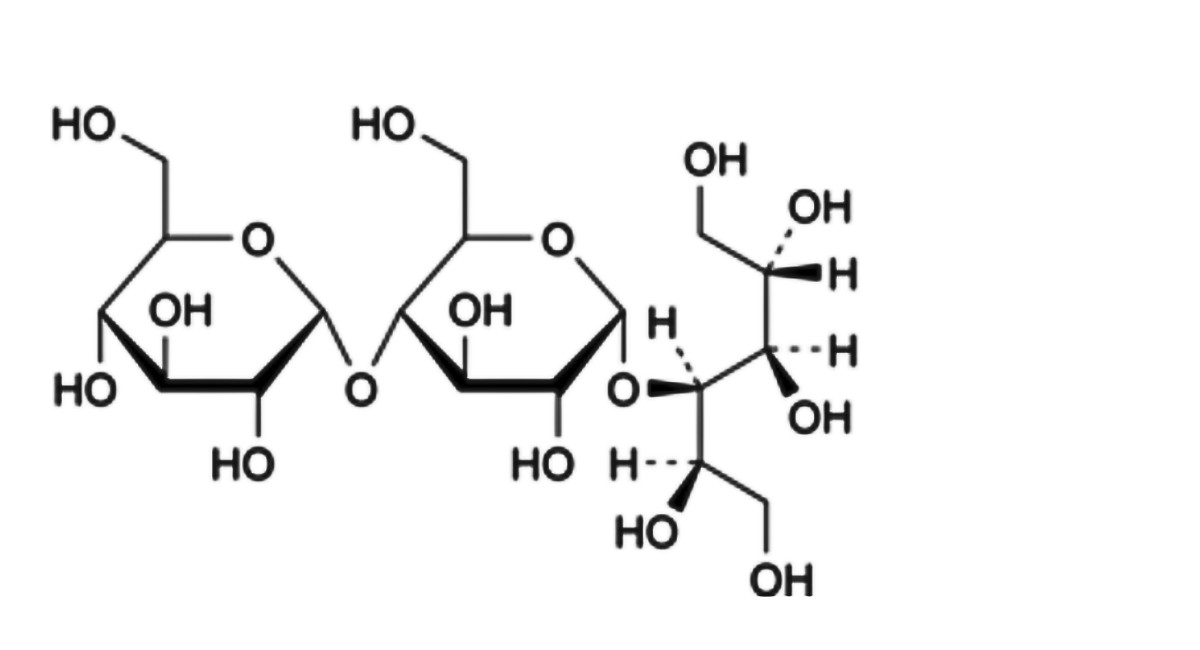
B. α-D-glucopyranosyl-(1→4)-α-D-glucopyranosyl-(1→4)-D-glucitol (maltotriitol).