Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 2080)
C4H6O5 134.1 6915-15-7
Action and use
Excipient.
DEFINITION
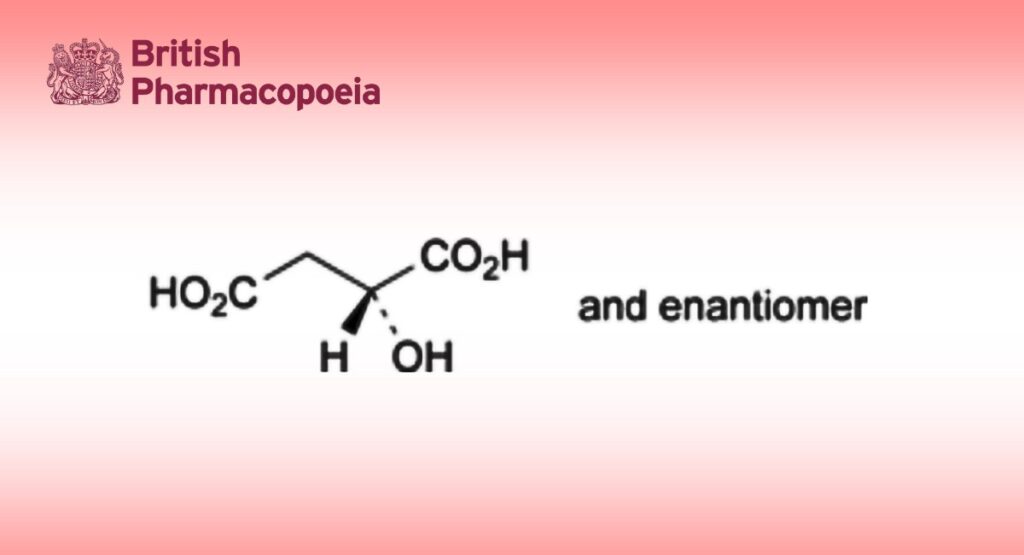
(2RS)-2-Hydroxybutanedioic acid.
Content
99.0 per cent to 101.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water and in ethanol (96 per cent), sparingly soluble in acetone.
IDENTIFICATION
A. Melting point (2.2.14): 128 °C to 132 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison Ph. Eur. reference spectrum of malic acid.
TESTS
Solution S
Dissolve 5.00 g in water R and dilute to 25 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Optical rotation (2.2.7)
-0.10° to + 0.10°, determined on solution S.
Water-insoluble substances
Maximum 0.1 per cent.
Dissolve 25.0 g in 100 mL of water R, filter the solution through a tared sintered-glass filter (16) (2.1.2), wash the filter with hot water R and dry at 100-105 °C to constant weight. The residue weighs a maximum of 25 mg.
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 100.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (a) Dissolve 10.0 mg of fumaric acid R and 4.0 mg of maleic acid R in 25 mL of the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (b) Dilute 2.5 mL of reference solution (a) to 100.0 mL with the mobile phase.
Reference solution (c) Dissolve 20.0 mg of the substance to be examined in the mobile phase, add 1.0 mL of reference solution (a) and dilute to 20.0 mL with the mobile phase.
Column:
— size: l = 0.30 m, Ø = 7.8 mm,
— stationary phase: ion-exclusion resin for chromatography R (9 μm),
— temperature: 37 °C.
Mobile phase 0.005 M sulfuric acid.
Flow rate 0.6 mL/min.
Detection Spectrophotometer at 210 nm.
Injection 20 μL.
Run time Twice the retention time of the principal peak in the chromatogram obtained with the test solution.
Relative retention With reference to malic acid (retention time = about 10 min): impurity B = about 0.8; impurity A = about 1.5.
System suitability Reference solution (c):
— resolution: minimum 2.5 between the peaks due to impurity B and malic acid.
Limits:
— impurity A: not more than twice the area of the corresponding peak in the chromatogram obtained with reference solution (b) (1.0 per cent),
— impurity B: not more than 0.25 times the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.05 per cent),
— any other impurity: for each impurity, not more than 0.5 times the area of the peak due to impurity B in the chromatogram obtained with reference solution (b) (0.1 per cent),
— total of other impurities: not more than 2.5 times the area of the peak due to impurity B in the chromatogram obtained with reference solution (b) (0.5 per cent),
— disregard limit: 0.1 times the area of the peak due to impurity B in the chromatogram obtained with reference solution (b) (0.02 per cent).
Water (2.5.12)
Maximum 2.0 per cent, determined on 1.00 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.500 g in 50 mL of carbon dioxide-free water R. Titrate with 1 M sodium hydroxide determining the end-point potentiometrically (2.2.20).
1 mL of 1 M sodium hydroxide is equivalent to 67.05 mg of C4H6O5
IMPURITIES
Specified impurities A, B.
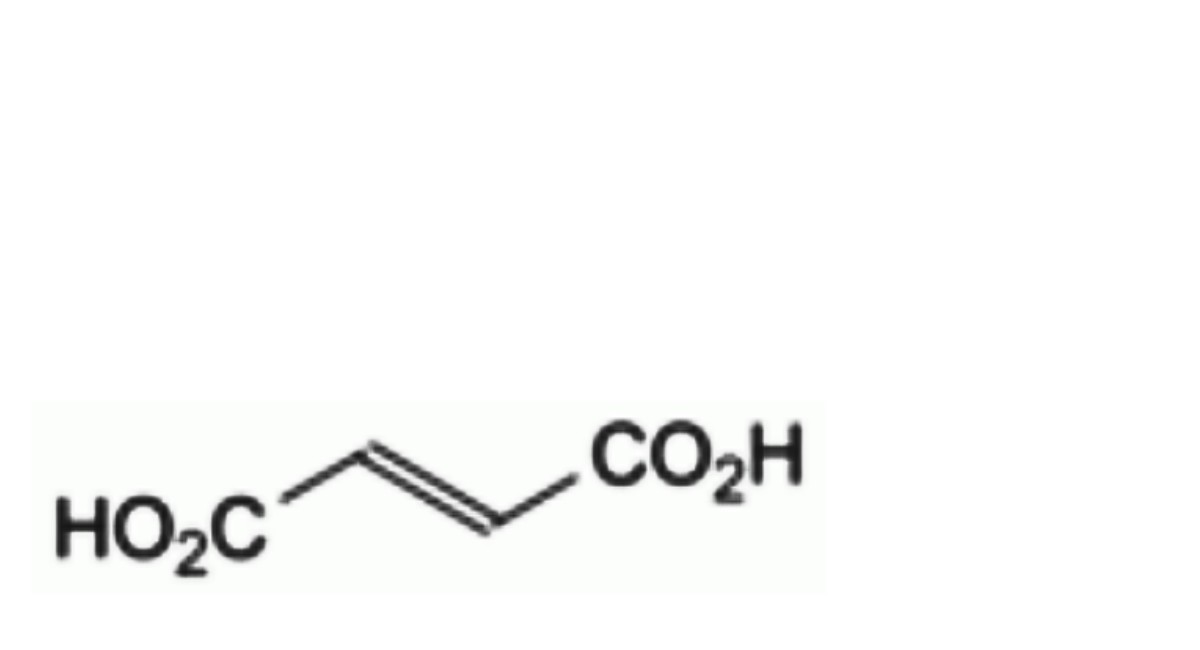
A. (E)-butenedioic acid (fumaric acid),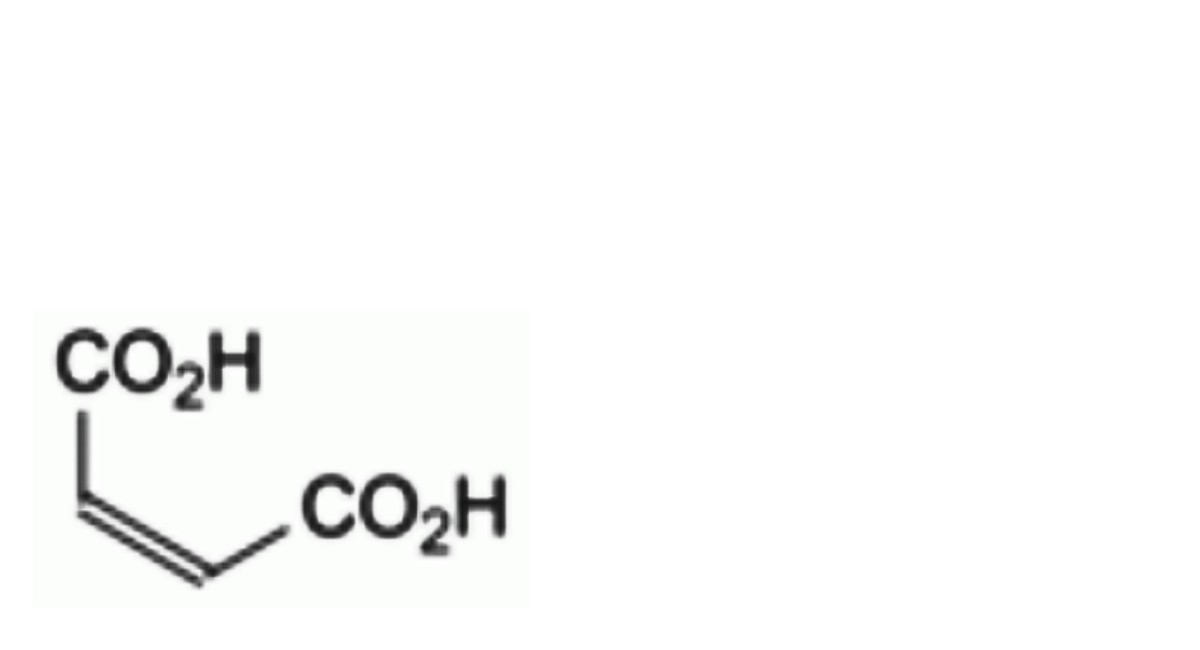
B. (Z)-butenedioic acid (maleic acid).