Edition: BP 2025 (Ph. Eur. 11.6 update)
(Ph. Eur. monograph 0365)
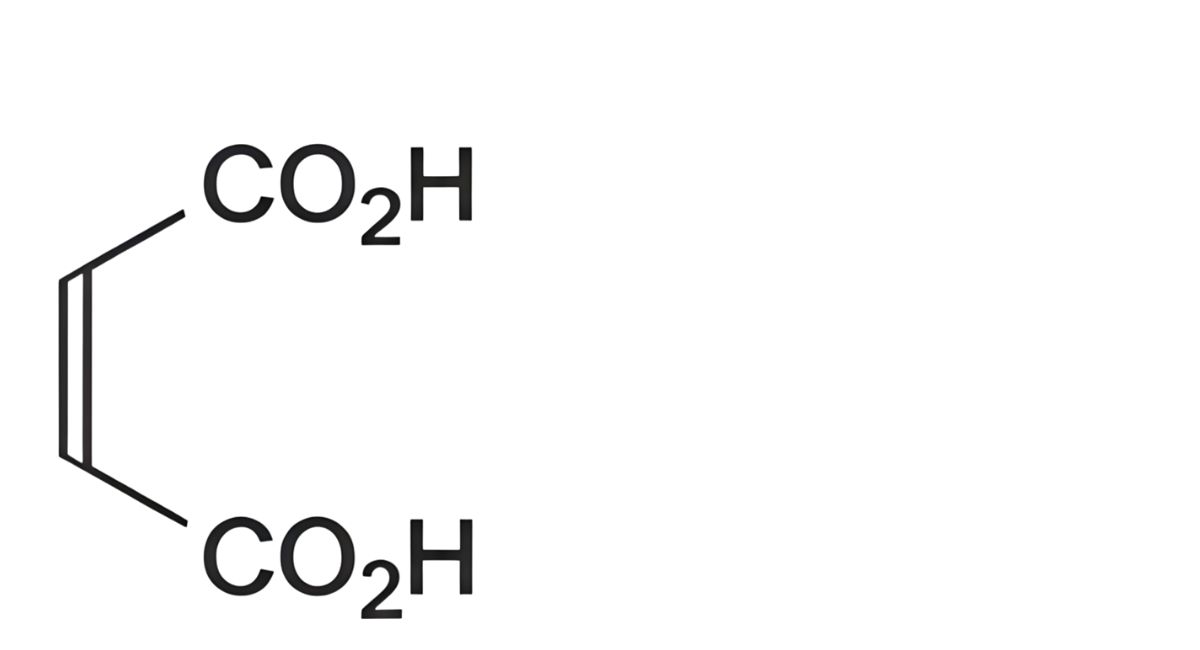
C4H4O4 116.1 110-16-7
Ph Eur
DEFINITION
Maleic acid contains not less than 99.0 per cent and not more than the equivalent of 101.0 per cent of (Z)- butenedioic acid, calculated with reference to the anhydrous substance.
CHARACTERS
A white or almost white, crystalline powder, freely soluble in water and in ethanol (96 per cent).
IDENTIFICATION
A. Dilute 5 mL of solution S (see Tests) to 10 mL with water R. The pH of the dilution is less than 2.
B. Examine the chromatograms obtained in the test for fumaric acid. The principal spot in the chromatogram obtained with test solution (b) is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Dissolve 0.1 g in 10 mL of water R (solution a). To 0.3 mL of solution (a) add a solution of 10 mg of resorcinol R in 3 mL of sulfuric acid R. Heat on a water-bath for 15 min; no colour develops. To 3 mL of solution (a) add 1 mL of bromine water R. Heat on a water-bath to remove the bromine (15 min), heat to boiling and cool. To 0.2 mL of this solution add a solution of 10 mg of resorcinol R in 3 mL of sulfuric acid R. Heat on a water-bath for 15 min. A violet-pink colour develops.
TESTS
Solution S
Dissolve 5.0 g in water R and dilute to 50 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y7 (2.2.2, Method II).
Fumaric acid
Examine by thin-layer chromatography (2.2.27), using silica gel GF254 R as the coating substance.
Test solution (a) Dissolve 0.5 g of the substance to be examined in acetone R and dilute to 5 mL with the same solvent.
Test solution (b) Dilute 1 mL of test solution (a) to 50 mL with acetone R.
Reference solution (a) Dissolve 20 mg of maleic acid CRS in acetone R and dilute to 10 mL with the same solvent.
Reference solution (b) Dissolve 15 mg of fumaric acid CRS in acetone R and dilute to 10 mL with the same solvent.
Reference solution (c) Mix 5 mL of reference solution (a) and 5 mL of reference solution (b).
Apply separately to the plate 5 μL of test solutions (a) and (b), 5 μL of reference solutions (a) and (b) and 10 μL of reference solution (c). Develop in an unsaturated tank over a path of 10 cm using a mixture of 12 volumes of anhydrous formic acid R, 16 volumes of chloroform R, 32 volumes of butanol R and 44 volumes of heptane R. Dry the plate at 100 °C for 15 min and examine in ultraviolet light at 254 nm. Any spot corresponding to fumaric acid in the chromatogram obtained with test solution (a) is not more intense than the spot in the chromatogram obtained with reference solution (b) (1.5 per cent). The test is not valid unless the chromatogram obtained with reference solution (c) shows two clearly separated spots.
Iron
To 10 mL of solution S add 2 mL of dilute hydrochloric acid R and 0.05 mL of bromine water R. After 5 min, remove the excess of bromine by passing a current of air and add 3 mL of potassium thiocyanate solution R. Shake. Prepare a standard at the same time and in the same manner, using a mixture of 5 mL of iron standard solution (1 ppm Fe) R, 1 mL of dilute hydrochloric acid R, 6 mL of water R and 0.05 mL of bromine water R. Allow both solutions to stand for 5 min. Any red colour in the test solution is not more intense than that in the standard (5 ppm).
Water (2.5.12)
Not more than 2.0 per cent, determined on 1.00 g by the semi-micro determination of water.
Sulfated ash (2.4.14)
Not more than 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.500 g in 50 mL of water R. Titrate with 1 M sodium hydroxide using 0.5 mL of phenolphthalein solution R as indicator.
1 mL of 1 M sodium hydroxide is equivalent to 58.04 mg of C4H4O4
STORAGE
Store in a glass container, protected from light.
Ph Eur