Edition: BP 2025 (Ph. Eur. 11.6 update)
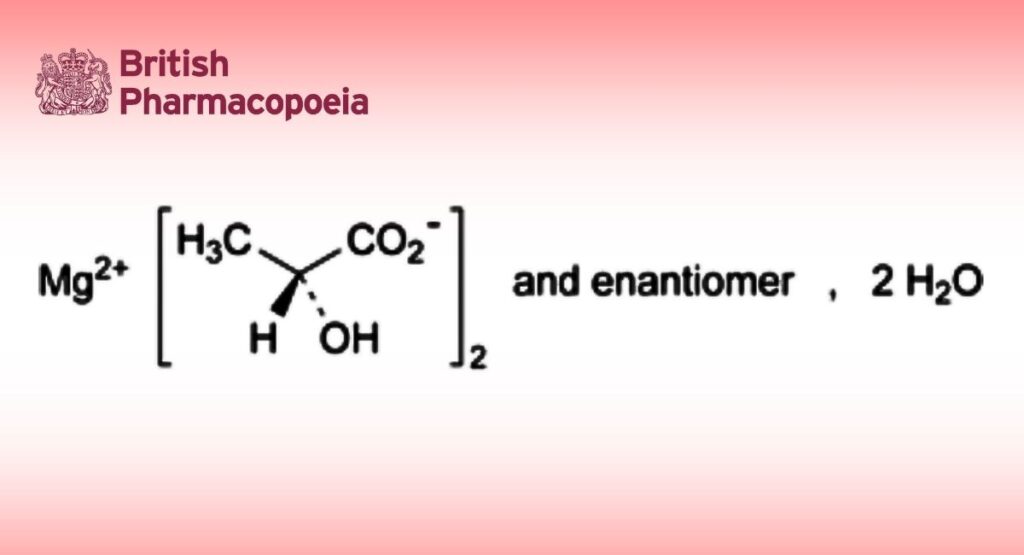
C6H10MgO6,2H2O 238.5
DEFINITION
Magnesium bis(2-hydroxypropanoate) or mixture of magnesium (2R)-, (2S)- and (2RS)-2- hydroxypropanoate dihydrate.
Content
98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline or granular powder.
Solubility
Slightly soluble in water, soluble in boiling water, practically insoluble in ethanol (96 per cent).
IDENTIFICATION
A. It gives the reaction of lactates (2.3.1).
B. It gives the reaction of magnesium (2.3.1).
TESTS
Solution S
Dissolve 5.0 g with heating in carbon dioxide-free water R prepared from distilled water R, allow to cool and
dilute to 100 mL with the same solvent.
Appearance of solution
Solution S is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than
reference solution BY6 (2.2.2, Method II).
pH (2.2.3)
6.5 to 8.5 for solution S.
Chlorides (2.4.4)
Maximum 200 ppm.
Dilute 5 mL of solution S to 15 mL with water R.
Sulfates (2.4.13)
Maximum 400 ppm.
Dilute 7.5 mL of solution S to 15 mL with distilled water R.
Iron (2.4.9)
Maximum 50 ppm.
Dilute 4 mL of solution S to 10 mL with water R.
Loss on drying (2.2.32)
14.0 per cent to 17.0 per cent, determined on 0.500 g by drying in an oven at 125 °C.
ASSAY
Dissolve 0.180 g in water R and dilute to 300 mL with the same solvent. Carry out the complexometric titration of magnesium (2.5.11).
1 mL of 0.1 M sodium edetate is equivalent to 20.25 mg of C6H10MgO6