Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
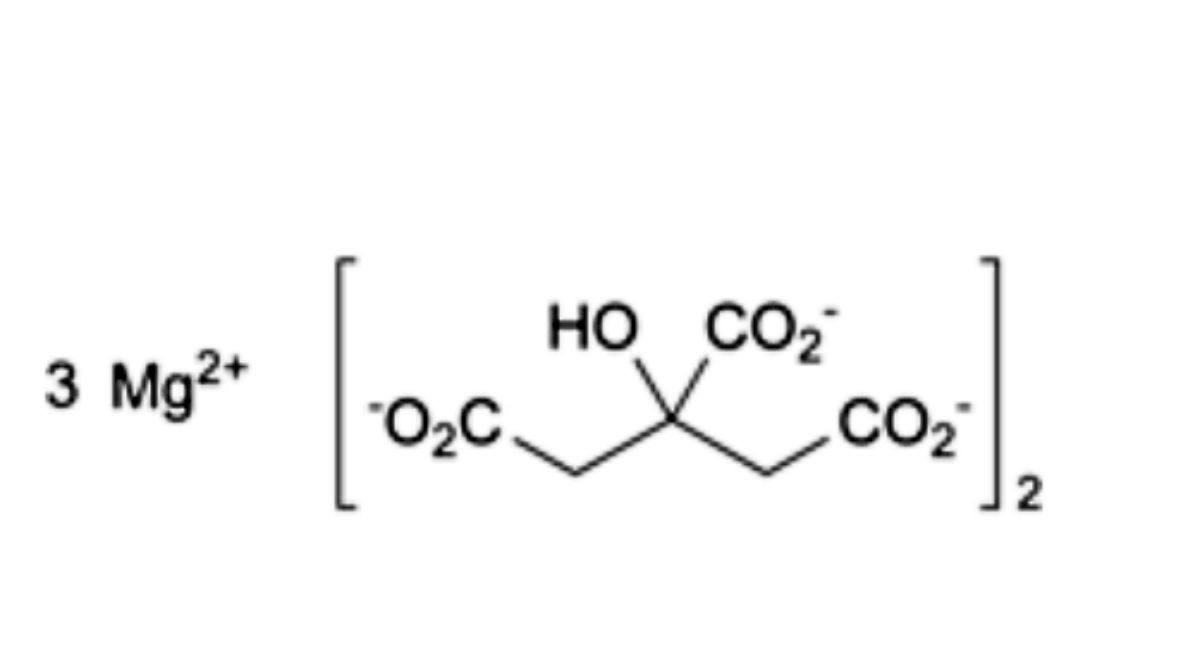
Anhydrous Magnesium Citrate
(Ph. Eur. monograph 2339)
Mg3(C6H5O7)2 451.1 3344-18-1
DEFINITION
Trimagnesium bis(2-hydroxypropane-1,2,3-tricarboxylate).
Content
15.0 per cent to 16.5 per cent of Mg (dried substance).
CHARACTERS
Appearance
White or almost white, fine, slightly hygroscopic powder.
Solubility
Soluble in water, practically insoluble in ethanol (96 per cent). It dissolves in dilute hydrochloric acid.
IDENTIFICATION
A. It gives the reaction of citrates (2.3.1).
B. It gives the reaction of magnesium (2.3.1).
C. pH (see Tests).
D. Loss on drying (see Tests).
TESTS
Solution S
Dissolve 5.0 g in carbon dioxide-free water R, heating at 60 °C, cool and dilute to 100 mL with the same solvent.
Appearance of solution
Solution S is not more opalescent than reference suspension III (2.2.1) and not more intensely coloured than reference solutions Y7 or BY6 (2.2.2, Method II).
pH (2.2.3)
6.0 to 8.5 for solution S.
Oxalates
Maximum 280 ppm.
Dissolve 0.50 g in 4 mL of water R. Add 3 mL of hydrochloric acid R and 1 g of activated zinc R. Allow to stand for 5 min. Transfer the liquid to a tube containing 0.25 mL of a 10 g/L solution of phenylhydrazine hydrochloride R. Heat to boiling. Cool rapidly, transfer to a graduated cylinder and add an equal volume of hydrochloric acid R and 0.25 mL of potassium ferricyanide solution R. Shake and allow to stand for 30 min. Any pink colour in the solution is not more intense than that in a standard prepared at the same time and in the same manner using 4 mL of a 50 mg/L solution of oxalic acid R.
Sulfates (2.4.13)
Maximum 0.2 per cent.
Dilute 1.5 mL of solution S to 15 mL with distilled water R.
Calcium (2.4.3)
Maximum 0.2 per cent.
Dilute 1.0 mL of solution S to 15 mL with distilled water R.
Iron (2.4.9)
Maximum 100 ppm.
Dilute 2.0 mL of solution S to 10 mL with distilled water R.
Loss on drying (2.2.32)
Maximum 3.5 per cent, determined on 1.000 g by drying in an oven at 180 ± 10 °C for 5 h.
ASSAY
Dissolve 0.150 g in 50 mL of waterR. Carry out the complexometric titration of magnesium (2.5.11).
1 mL of 0.1 M sodium edetate is equivalent to 2.431 mg of Mg.
STORAGE
In a non-metallic, airtight container.






