BP 2025 (Ph. Eur. 11.6 update)
DEFINITION
Grafted copolymer of macrogol and poly(vinyl alcohol), having a mean relative molecular mass of about 45 000.
It consists of about 75 per cent of poly(vinyl alcohol) units and 25 per cent of macrogol units. It may contain Anhydrous colloidal silica (0434) to improve flowability.
CHARACTERS
Appearance
White or slightly yellowish powder; opalescent solutions may be obtained during testing due to the presence of anhydrous colloidal silica.
Solubility
Very soluble in water, practically insoluble in anhydrous ethanol and in acetone. It dissolves in dilute acids and in dilute solutions of alkali hydroxides.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24). Comparison macrogol poly(vinyl alcohol) grafted copolymer CRS. Preparation Dissolve 0.2 g in 20 mL of water R, spread a few drops of the solution on a thallium bromoiodide plate and evaporate the solvent at 110 °C for 30 min.
B. Dissolve 0.4 g in 2 mL of water R. Place 1 mL of the solution on a glass plate and allow to dry. A transparent film is formed.
TESTS
pH (2.2.3)
5.0 to 8.0.
Dissolve 5.0 g in carbon dioxide-free water R and dilute to 25.0 mL with the same solvent.
Ester value
10 to 75.
Determine the acid value (IA) as follows. Dissolve 5.00 g in 100 mL of distilled water R while stirring with a magnetic stirrer. Titrate with 0.01 M alcoholic potassium hydroxide, determining the end-point potentiometrically (2.2.20). Carry out a blank test under the same conditions.
IA= 0.561(n1−n2)/m
n1 = volume of titrant used in the test, in millilitres;
n2 = volume of titrant used in the blank test, in millilitres;
m = mass of the sample, in grams.
Determine the saponification value (IS) (2.5.6) on 5.00 g, using 50.0 mL of 0.5 M alcoholic potassium hydroxide and stirring vigorously with a magnetic stirrer.
The ester value (IE) is calculated from the saponification value (IS) and the acid value (IA):
IE = IS − IA
Ethylene oxide and dioxan (2.4.25)
Maximum 1 ppm of ethylene oxide and 10 ppm of dioxan.
Impurity A
Liquid chromatography (2.2.29).
Test solution Introduce 0.250 g of the substance to be examined into a 10 mL volumetric flask and add about 1 mL of methanol R. Sonicate. Add about 8 mL of water R and dilute to 10.0 mL with the same solvent. Filter.
Reference solution (a) Dissolve 5.0 mg of vinyl acetate CRS (impurity A) in methanol R and dilute to 10.0 mL with the same solvent. Dilute 1.0 mL of the solution to 20.0 mL with water R. Dilute 1.0 mL of this solution to 10.0 mL with water R.
Reference solution (b) Dissolve 5 mg of vinyl acetate R (impurity A) and 5 mg of 1-vinylpyrrolidin-2-one R in 10 mL of methanol R and dilute to 50 mL with water R. Dilute 1 mL of the solution to 20 mL with water R.
A precolumn containing end-capped octadecylsilyl silica gel for chromatography R (5 μm) may be used if a matrix effect is observed.
Column:
— size: l = 0.25 m, Ø = 4.0 mm;
— stationary phase: polar end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase:
— mobile phase A: acetonitrile R1, methanol R2, water for chromatography R (5:5:90 V/V/V);
— mobile phase B: methanol R2, acetonitrile R1, water for chromatography R (5:45:50 V/V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 2 | 100 | 0 |
| 2 – 40 | 100 → 85 | 0 → 15 |
| 40 – 42 | 85 → 0 | 15 → 100 |
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 205 nm.
Injection 10 μL.
Retention time Impurity A = about 19 min; 1-vinylpyrrolidin-2-one = about 25 min.
System suitability Reference solution (b):
— resolution: minimum 5.0 between the peaks due to impurity A and 1-vinylpyrrolidin-2-one.
Limit:
— impurity A: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (100 ppm).
Impurity B
Liquid chromatography (2.2.29).
Test solution Mix 0.200 g of the substance to be examined with water R and dilute to 10.0 mL with the same solvent.
Reference solution Dissolve 30 mg of citric acid monohydrate R and 0.100 g of acetic acid R (impurity B) in the mobile phase. Shake gently to dissolve and dilute to 100.0 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: polar end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase 0.50 g/L solution of sulfuric acid R.
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 205 nm.
Injection 20 μL. After each injection, rinse the column with a mixture of equal volumes of acetonitrile R1 and a 0.50 g/L solution of sulfuric acid R.
Retention time Impurity B = about 5 min; citric acid = about 7 min.
System suitability Reference solution:
— resolution: minimum 2.0 between the peaks due to impurity B and citric acid.
Limit:
— impurity B: not more than the area of the corresponding peak in the chromatogram obtained with the reference solution (1.5 per cent).
Sulfated ash (2.4.14)
Maximum 3.0 per cent, determined on 5.0 g.
Loss on drying (2.2.32)
Maximum 5.0 per cent, determined on 1.000 g by drying in vacuo at 105 °C.
IMPURITIES
Specified impurities A, B.
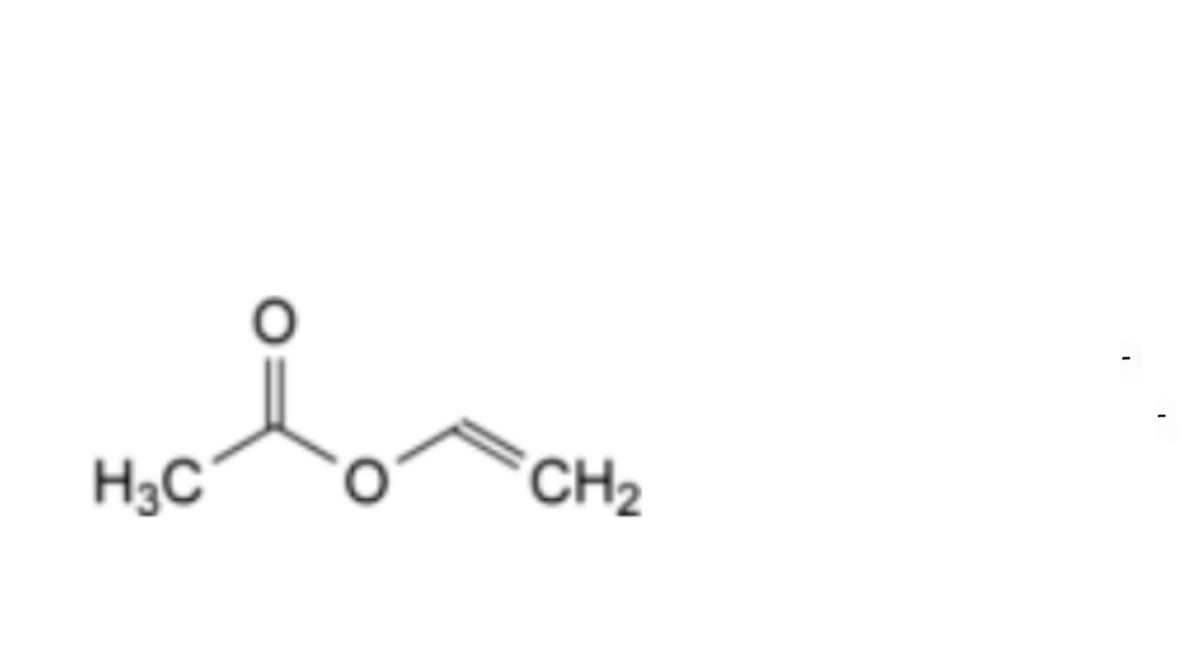
A. ethenyl acetate
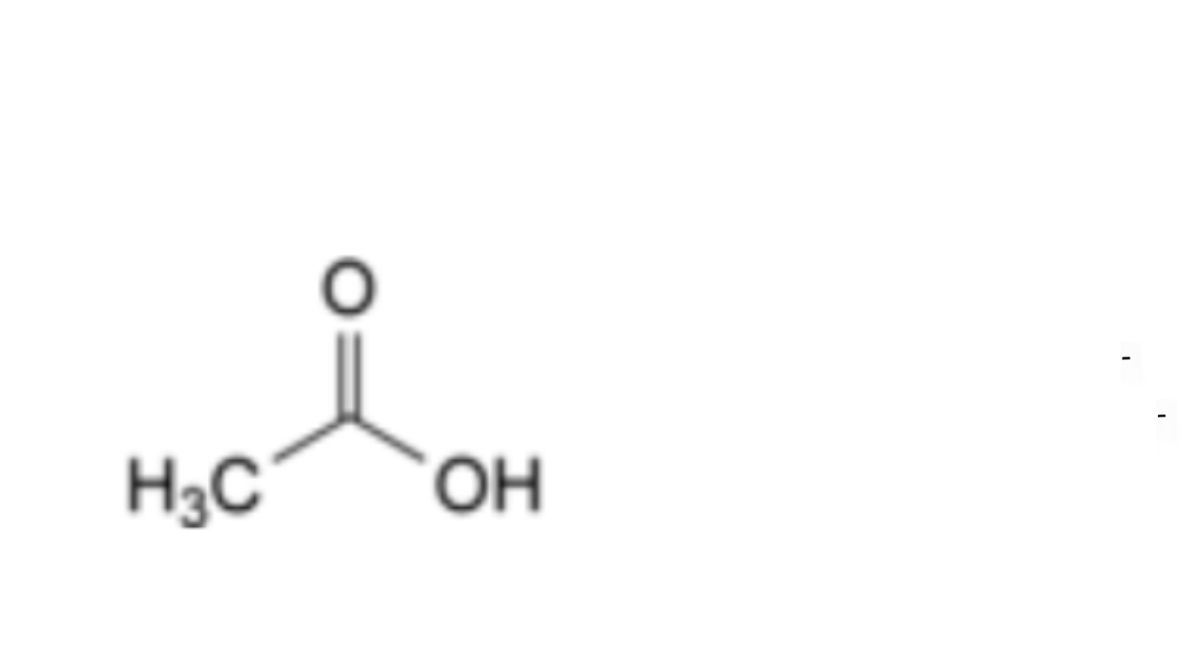
B. acetic acid.
FUNCTIONALITY-RELATED CHARACTERISTICS
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient (see chapter 5.15). Some of the characteristics described in the Functionality-related characteristics section may also be present in the mandatory part of the monograph since they also represent mandatory quality criteria. In such cases, a
cross-reference to the tests described in the mandatory part is included in the Functionality-related characteristics section. Control of the characteristics can contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.
The following characteristics may be relevant for macrogol poly(vinyl alcohol) grafted copolymer used as film former in film-coated tablets.
Viscosity (2.2.10)
Typically less than 250 mPa·s, determined on a 20 per cent m/m solution, using a rotating viscometer at 25 °C and rotation speed of 100 r/min.






