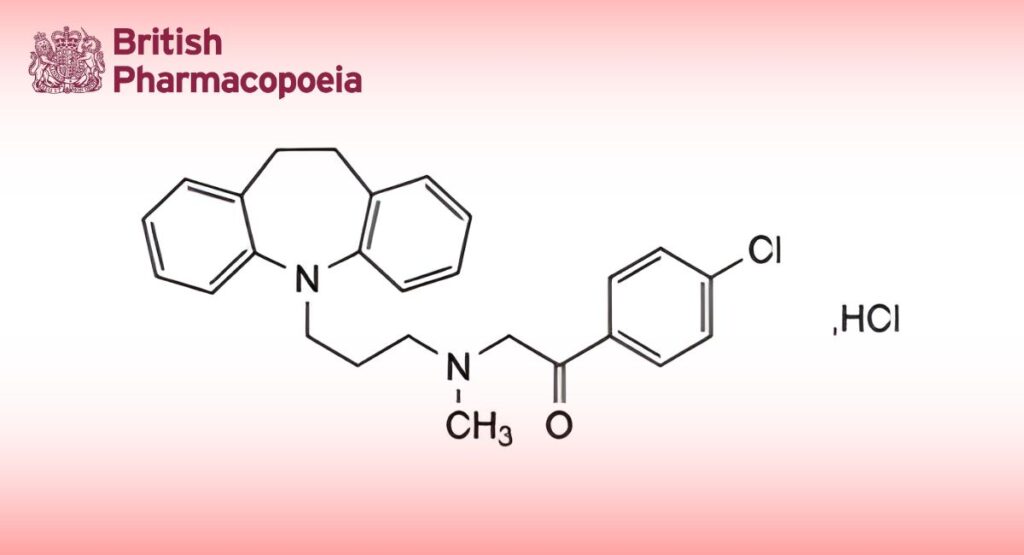
C26H27ClN2O,HCl 455.4 26786-32-3
Action and use
Monoamine reuptake inhibitor; tricyclic antidepressant.
Preparation
Lofepramine Tablets
DEFINITION
Lofepramine Hydrochloride is 5-(3-[N-(4-chlorophenacyl)-N-methylamino]propyl)10,11-dihydro-5H-
dibenz[b,f]azepine hydrochloride. It contains not less than 98.5% and not more than 101.0% of C26H27ClN2O,HCl, calculated with reference to the dried substance.
CHARACTERISTICS
A fine, yellowish white to greenish yellow powder.
Very soluble in ethanol (96%) and in methanol; slightly soluble in acetone; very slightly soluble in water. It exhibits polymorphism.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of
lofepramine hydrochloride (form A) (RS 399A).
B. 2 mL of a 1% w/v solution in ethanol (96%) complies with the test for chlorides, Appendix VI.
TESTS
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Solution (1) contains 0.1% w/v of the substance being examined in the mobile phase. For solution (2) dilute 1 volume of solution (1) to 200 volumes with the mobile phase. Solution (3) contains 0.0002% w/v each of desipramine hydrochloride BPCRS and imipramine hydrochloride BPCRS in the mobile phase.
Inject 20 μL of solutions (1) and (2). Inject 20 μL of solution (3) and allow the chromatography to continue for 4 times the retention time of the principal peak.
The chromatographic procedure may be carried out using (a) a stainless steel column (25 cm × 4.6 mm) packed with base-deactivated end-capped octylsilyl silica gel for chromatography (5 μm) (Lichrospher 60 RP-select B is suitable) maintained at 50°, (b) as the mobile phase with a flow rate of 1.5 mL per minute a 0.09% w/v solution of sodium dodecyl sulfate in a mixture of 550 volumes of acetonitrile, 325 volumes of water and 125 volumes of a buffer solution of pH 1.0 containing 0.015% w/v of glycine, 0.018% w/v of sodium chloride and 0.44% w/v of hydrochloric acid and (c) a detection wavelength of 254 nm.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the two principal peaks is at least 0.9.
In the chromatogram obtained with solution (1) the area of any secondary peak is not greater than the area of the peak in the chromatogram obtained with solution (2) (0.5%) and the sum of the areas of any
secondary peaks is not greater than twice the area of the peak in the chromatogram obtained with solution
(2) (1%).
Loss on drying
When dried at a temperature of 100° at a pressure not exceeding 0.2 kPa, loses not more than 0.5% of its
weight. Use 1 g.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Solution (1) contains 0.02% w/v of the substance being examined in the mobile phase. Solution (2) contains 0.02% w/v of lofepramine hydrochloride BPCRS in the mobile phase.
The chromatographic procedure described under the test for Related substances may be used.
Inject 20 μL of each solution.
Calculate the content of C26H27ClN2O,HCl from the chromatograms obtained and using the declared content of C26H27ClN2O,HCl in lofepramine hydrochloride BPCRS.
STORAGE
Lofepramine Hydrochloride should be kept in an airtight container and protected from light.
IMPURITIES
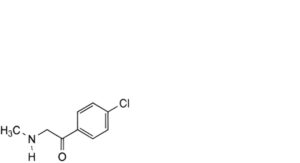
A. 1-methylamino-4′-chloroacetophenone,
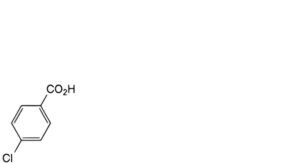
B. 4-chlorobenzoic acid,
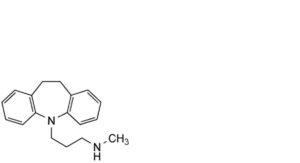
C. desipramine,
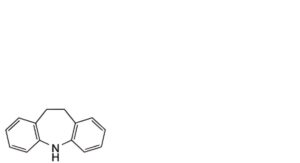
D. iminodibenzyl,
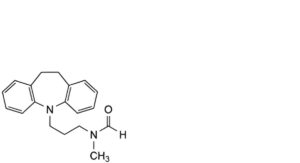
E. N-formyldesipramine,
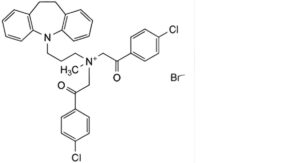
F. N,N-bis(4′-chlorophenacyl)desipramine bromide,
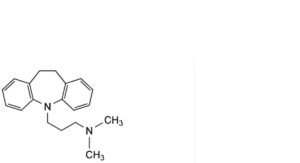
G. imipramine,
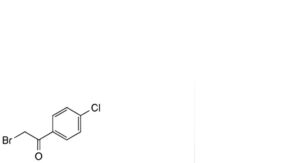
H. 2-bromo-4′-chloroacetophenone.