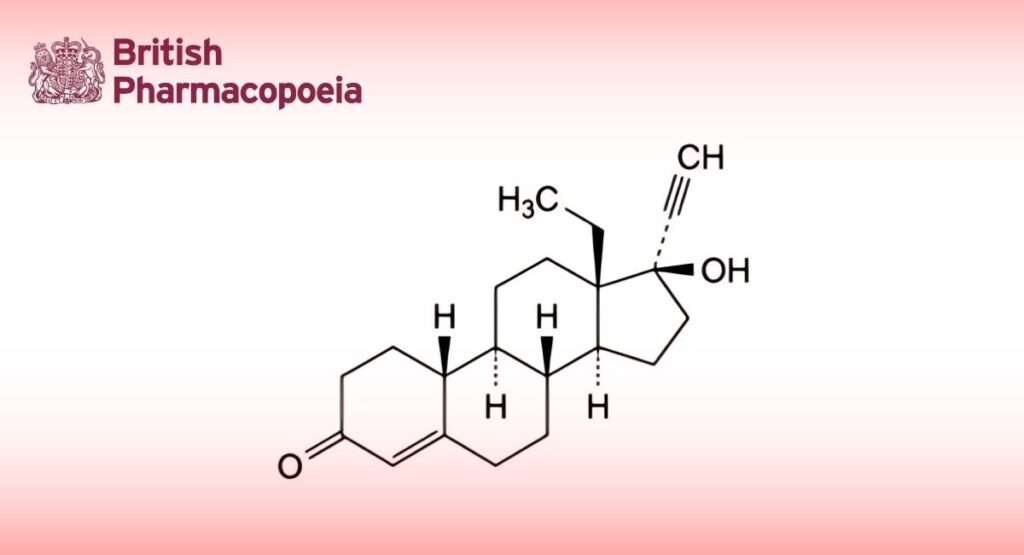
(Ph. Eur. monograph 0926)
C21H28O2 312.5 797-63-7
Action and use
Progestogen.
Preparations
Levonorgestrel Tablets
Levonorgestrel and Ethinylestradiol Tablets
DEFINITION
13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one.
Content
98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, sparingly soluble in methylene chloride, slightly soluble in ethanol (96 per cent).
IDENTIFICATION
A. Specific optical rotation (see Tests).
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: levonorgestrel CRS.
TESTS
Specific optical rotation (2.2.7)
-35 to -30.
Dissolve 0.200 g in methylene chloride R and dilute to 20.0 mL with the same solvent.
Related substances
A. Impurities A, B, H, K, M, O, S, U. Liquid chromatography (2.2.29).
Solvent mixture water R, acetonitrile R (30:70 V/V).
Test solution: Dissolve 10.0 mg of the substance to be examined in 7 mL of acetonitrile R using sonication and dilute to 10.0 mL with water R.
Reference solution (a): Dissolve 5 mg of levonorgestrel for system suitability 1 CRS (containing impurities A, H, K, M, O and S) in 3.5 mL of acetonitrile R using sonication and dilute to 5 mL with water R.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (c): Dissolve 5.0 mg of levonorgestrel impurity B CRS in 35 mL of acetonitrile R and dilute to 50.0 mL with water R. Dilute 1.0 mL of the solution to 100.0 mL with the solvent mixture.
Reference solution (d): Dissolve 5.0 mg of norethisterone CRS (impurity U) in 35 mL of acetonitrile R and dilute to 50.0 mL with water R. Dilute 1.0 mL of the solution to 100.0 mL with the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octylsilyl silica gel for chromatography with embedded polar groups R (5 μm);
— temperature: 30 °C.
Mobile phase:
— mobile phase A: acetonitrile R1, water for chromatography R (40:60 V/V);
— mobile phase B: acetonitrile R1;
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 50 | 100 → 20 | 0 → 80 |
Flow rate: 0.7 mL/min.
Detection: Spectrophotometer at 215 nm and, for impurity O, at 200 nm.
Injection: 50 μL.
Identification of impurities: Use the chromatograms supplied with levonorgestrel for system suitability 1 CRS and the chromatograms obtained with reference solution (a) at 215 nm to identify the peaks due to impurities A, H, K, M and S, and at 200 nm to identify the peak due to impurity O; use the chromatogram obtained with reference solution (c) to identify the peak due to impurity B; use the chromatogram obtained with reference solution (d) to identify the peak due to impurity U.
Relative retention: With reference to levonorgestrel (retention time = about 20 min): impurity H = about 0.5; impurity U = about 0.8; impurity K = about 0.85; impurity A = about 0.91; impurity M = about 0.95; impurity O = about 1.16; impurity B = about 1.26; impurity S = about 1.9.
System suitability:
— signal-to-noise ratio: minimum 60 for the principal peak in the chromatogram obtained with reference solution (b);
— peak-to-valley ratio: minimum 3.0, where Hp = height above the baseline of the peak due to impurity M and Hv = height above the baseline of the lowest point of the curve separating this peak
from the peak due to impurity A in the chromatogram obtained with reference solution (a).
Calculation of percentage contents:
— correction factors: multiply the peak areas of the following impurities by the corresponding correction factor: impurity A = 0.4; impurity M = 3.1; impurity O = 2.6;
— for impurity B, use the concentration of impurity B in reference solution (c);
— for impurity U, use the concentration of impurity U in reference solution (d);
— for impurities other than B and U, use the concentration of levonorgestrel in reference solution (b).
Limits:
— impurities A, B, K: for each impurity, maximum 0.3 per cent;
— impurity O at 200 nm: maximum 0.3 per cent;
— impurities M, S, U: for each impurity, maximum 0.2 per cent;
— impurity H: maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— sum of impurities other than O: maximum 1.0 per cent;
— reporting threshold: 0.05 per cent.
B. Impurities V and W. Liquid chromatography (2.2.29).
Solvent mixture water R, acetonitrile R (30:70 V/V).
Test solution: Dissolve 10.0 mg of the substance to be examined in 7 mL of acetonitrile R using sonication and dilute to 10.0 mL with water R.
Reference solution (a): Dissolve 5 mg of levonorgestrel for system suitability 2 CRS (containing impurities V and W) in 3.5 mL of acetonitrile R using sonication and dilute to 5 mL with water R.
Reference solution (b): Dissolve 5.0 mg of ethinylestradiol CRS in 35 mL of acetonitrile R using sonication and dilute to 50.0 mL with water R. Dilute 3.0 mL of the solution to 100.0 mL with the solvent mixture.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography compatible with 100 per cent aqueous mobile phases R (3 μm).
Mobile phase:
— mobile phase A: acetonitrile R1, water for chromatography R (40:60 V/V);
— mobile phase B: water for chromatography R, acetonitrile R1 (10:90 V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 1 | 92 | 8 |
| 1 – 3 | 92 → 82 | 8 → 18 |
| 3 – 6 | 82 | 18 |
| 6 – 16 | 82 → 60 | 18 → 40 |
| 16 – 21 | 60 → 0 | 40 → 100 |
| 21 – 32 | 0 | 100 |
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 200 nm.
Injection: 50 μL.
Identification of impurities: Use the chromatogram supplied with levonorgestrel for system suitability 2 CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to impurities V and W.
Relative retention: With reference to levonorgestrel (retention time = about 12 min): impurity W = about 0.9; impurity V = about 1.9.
System suitability: Reference solution (a):
— resolution: minimum 2.8 between the peaks due to impurity W and levonorgestrel.
Calculation of percentage contents:
— for each impurity, use the concentration of ethinylestradiol in reference solution (b).
Limits:
— impurity W: maximum 0.3 per cent;
— impurity V: maximum 0.15 per cent.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.200 g in 45 mL of tetrahydrofuran R. Add 10 mL of a 100 g/L solution of silver nitrate R. After 1 min, titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20).
Carry out a blank titration.
1 mL of 0.1 M sodium hydroxide is equivalent to 31.25 mg of C21H28O2.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, H, K, M, O, S, U, V, W.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.
Control of impurities in substances for pharmaceutical use) C, D, G, I, J, L, N, P, Q, R, T.
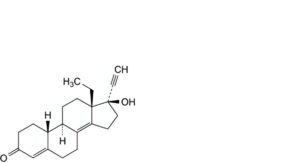
A. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregna-4,8(14)-dien-20-yn-3-one,
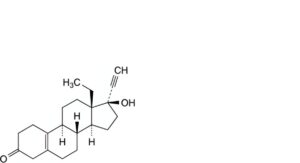
B. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-5(10)-en-20-yn-3-one,
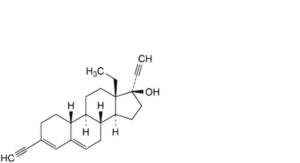
C. 13-ethyl-3-ethynyl-18,19-dinor-17α-pregna-3,5-dien-20-yn-17-ol,
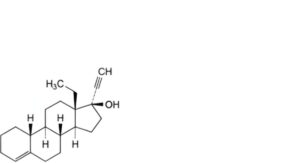
D. 13-ethyl-18,19-dinor-17α-pregn-4-en-20-yn-17-ol (3-deoxolevonorgestrel),
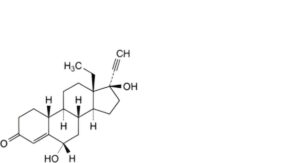
G. 13-ethyl-6α,17-dihydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one (6α-hydroxylevonorgestrel),
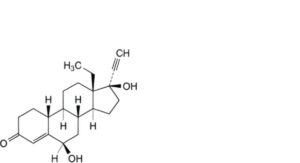
H. 13-ethyl-6β,17-dihydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one (6β-hydroxylevonorgestrel),
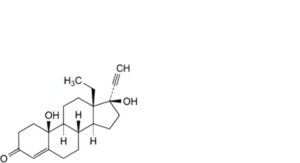
I. 13-ethyl-10,17-dihydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one (10-hydroxylevonorgestrel),
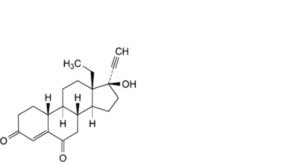
J. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yne-3,6-dione (6-oxolevonorgestrel),
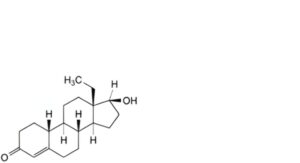
K. 13-ethyl-17β-hydroxygon-4-en-3-one (18-methylnandrolone),
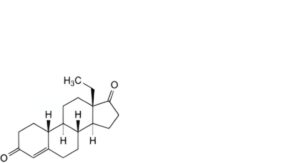
L. 13-ethylgon-4-ene-3,17-dione (levodione),
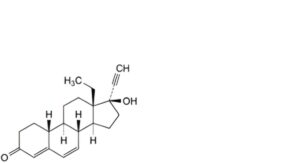
M. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregna-4,6-dien-20-yn-3-one (Δ6-levonorgestrel),
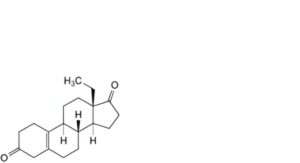
N. 13-ethylgon-5(10)-ene-3,17-dione (Δ5(10)-levodione),
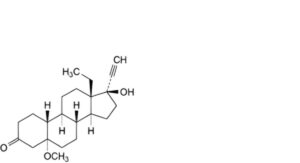
O. 13-ethyl-17-hydroxy-5α-methoxy-18,19-dinor-17α-pregn-20-yn-3-one (4,5-dihydro-5α-methoxylevonorgestrel),
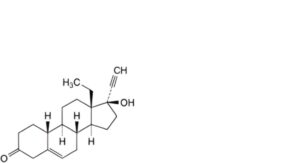
P. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-5-en-20-yn-3-one (Δ5-levonorgestrel),
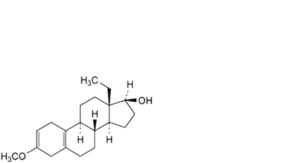
Q. 13-ethyl-3-methoxygona-2,5(10)-dien-17β-ol,
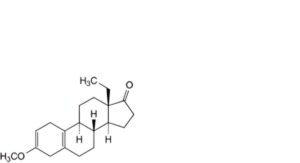
R. 13-ethyl-3-methoxygona-2,5(10)-dien-17-one,
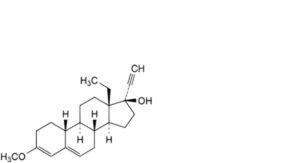
S. 13-ethyl-3-methoxy-18,19-dinor-17α-pregna-3,5-dien-20-yn-17-ol,
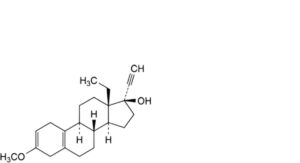
T. 13-ethyl-3-methoxy-18,19-dinor-17α-pregna-2,5(10)-dien-20-yn-17-ol,
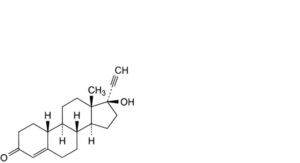
U. 17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one (norethisterone),
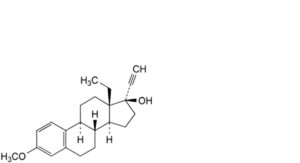
V. 13-ethyl-3-methoxy-18,19-dinor-17α-pregna-1,3,5(10)-trien-20-yn-17-ol,
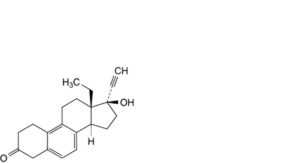
W. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregna-5,7,9-trien-20-yn-3-one.